3,3'-Diindolylmethane and Its Derivatives: Nature
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Molecular, Genetic, and Nutritional Aspects of Major and Trace Minerals
Molecular, Genetic, and Nutritional Aspects of Major and Trace Minerals Edited by James F. Collins AMSTERDAM • BOSTON • HEIDELBERG • LONDON • NEW ORK • OFORD • PARIS SAN DIEGO • SAN FRANCISCO • SINGAPORE • SDNE • TOKO Academic Press is an imprint of Elsevier Academic Press is an imprint of Elsevier 125 London Wall, London EC2Y 5AS, United Kingdom 525 B Street, Suite 1800, San Diego, CA 92101-4495, United States 50 Hampshire Street, 5th Floor, Cambridge, MA 02139, United States The Boulevard, Langford Lane, Kidlington, Oxford OX5 1GB, United Kingdom Copyright © 2017 Elsevier Inc. All rights reserved. No part of this publication may be reproduced or transmitted in any form or by any means, electronic or mechanical, including photocopying, recording, or any information storage and retrieval system, without permission in writing from the publisher. Details on how to seek permission, further information about the Publisher’s permissions policies and our arrangements with organizations such as the Copyright Clearance Center and the Copyright Licensing Agency, can be found at our website: www.elsevier.com/permissions. This book and the individual contributions contained in it are protected under copyright by the Publisher (other than as may be noted herein). Notices Knowledge and best practice in this field are constantly changing. As new research and experience broaden our understanding, changes in research methods, professional practices, or medical treatment may become necessary. Practitioners and researchers must always rely on their own experience and knowledge in evaluating and using any information, methods, compounds, or experiments described herein. In using such information or methods they should be mindful of their own safety and the safety of others, including parties for whom they have a professional responsibility. -

Assessment of NR4A Ligands That Directly Bind and Modulate the Orphan Nuclear Receptor Nurr1
bioRxiv preprint doi: https://doi.org/10.1101/2020.05.22.109017; this version posted May 25, 2020. The copyright holder for this preprint (which was not certified by peer review) is the author/funder, who has granted bioRxiv a license to display the preprint in perpetuity. It is made available under aCC-BY 4.0 International license. Assessment of NR4A Ligands that Directly Bind and Modulate the Orphan Nuclear Receptor Nurr1 Paola Munoz-Tello 1, Hua Lin 2,3, Pasha Khan 2, Ian Mitchelle S. de Vera 1,4, Theodore M. Kamenecka 2, and Douglas J. Kojetin 1,2,* 1 Department of Integrative Structural and Computational Biology, The Scripps Research Institute, Jupiter, FL, 33458, USA 2 Department of Molecular Medicine, The Scripps Research Institute, Jupiter, Florida 33458, USA 3 Current address: Biomedical Research Center of South China, College of Life Sciences, Fujian Normal University, Fuzhou 350117, China 4 Current address: Department of Pharmacology and Physiology, Saint Louis University School of Medicine, St. Louis, MO 63104, USA * Correspondence: [email protected] as ligands that increase Nurr1 transcription in human neuroblastoma SK- ABSTRACT N-BE(2)-C cells (11). Amodiaquine improves behavioral alterations in a Nurr1/NR4A2 is an orphan nuclear receptor transcription factor im- Parkinson’s disease animal model (11) and improves neuropathology and plicated as a potential drug target for neurological disorders including memory impairment in an Alzheimer’s disease animal model (12). Alzheimer’s and Parkinson’s diseases. Previous studies identified small molecule modulators of NR4A nuclear receptors including Nurr1 and Amodiaquine is the most potent and efficacious Nurr1 agonist of the Nur77/NR4A1; it remains unclear whether these ligands affect Nurr1 4-amino-7-chloroquinoline compounds, but these compounds are not through direct binding or indirect non-binding mechanisms. -

Supplementary Data Genbank Or OSE Vs RO NIA Accession Gene Name Symbol FC B-Value H3073C09 11.38 5.62 H3126B09 9.64 6.44 H3073B0
Supplementary Data GenBank or OSE vs RO NIA accession Gene name Symbol FC B-value H3073C09 11.38 5.62 H3126B09 9.64 6.44 H3073B08 9.62 5.59 AU022767 Exportin 4 Xpo4 9.62 6.64 H3073B09 9.59 6.48 BG063925 Metallothionein 2 Mt2 9.23 18.89 H3064B07 9.21 6.10 H3073D08 8.28 6.10 AU021923 Jagged 1 Jag1 7.89 5.93 H3070D08 7.54 4.58 BG085110 Cysteine-rich protein 1 (intestinal) Crip1 6.23 16.40 BG063004 Lectin, galactose binding, soluble 1 Lgals1 5.95 10.36 BG069712 5.92 2.34 BG076976 Transcribed locus, strongly similar to NP_032521.1 lectin, galactose binding, soluble 1 5.64 8.36 BG062930 DNA segment, Chr 11, Wayne State University 99, expressed D11Wsu99e 5.63 8.76 BG086474 Insulin-like growth factor binding protein 5 Igfbp5 5.50 15.95 H3002d11 5.13 20.77 BG064706 Keratin complex 1, acidic, gene 19 Krt1-19 5.06 9.07 H3007A09 5.05 2.46 H3065F02 4.84 5.43 BG081752 4.81 1.25 H3010E09 4.71 11.90 H3064c11 4.43 1.00 BG069711 Transmembrane 4 superfamily member 9 Tm4sf9 4.29 1.23 BG077072 Actin, beta, cytoplasmic Actb 4.29 3.01 BG079788 Hemoglobin alpha, adult chain 1 Hba-a1 4.26 6.63 BG076798 4.23 0.80 BG074344 Mesothelin Msln 4.22 6.97 C78835 Actin, beta, cytoplasmic Actb 4.16 3.02 BG067531 4.15 1.61 BG073468 Hemoglobin alpha, adult chain 1 Hba-a1 4.10 6.23 H3154H07 4.08 5.38 AW550167 3.95 5.66 H3121B01 3.94 5.94 H3124f12 3.94 5.64 BG073608 Hemoglobin alpha, adult chain 1 Hba-a1 3.84 5.32 BG073617 Hemoglobin alpha, adult chain 1 Hba-a1 3.84 5.75 BG072574 Hemoglobin alpha, adult chain 1 Hba-a1 3.82 5.93 BG072211 Tumor necrosis factor receptor superfamily, -

Rapid Effects of LH on Gene Expression in the Mural Granulosa Cells of Mouse Periovulatory Follicles
REPRODUCTIONRESEARCH Rapid effects of LH on gene expression in the mural granulosa cells of mouse periovulatory follicles Martha Z Carletti and Lane K Christenson Department of Molecular and Integrative Physiology, University of Kansas Medical Center, 3075 KLSIC, 3901 Rainbow Boulevard, Kansas City, Kansas 66160, USA Correspondence should be addressed to L K Christenson; Email: [email protected] Abstract LH acts on periovulatory granulosa cells by activating the PKA pathway as well as other cell signaling cascades to increase the transcription of specific genes necessary for ovulation and luteinization. Collectively, these cell signaling responses occur rapidly (within minutes); however, presently no high throughput studies have reported changes before 4 h after the LH surge. To identify early response genes that are likely critical for initiation of ovulation and luteinization, mouse granulosa cells were collected before and 1 h after hCG. Fifty-seven gene transcripts were significantly (P!0.05) upregulated and three downregulated following hCG. Twenty-four of these transcripts were known to be expressed after the LH/hCG surge at later time points, while 36 were unknown to be expressed by periovulatory granulosa cells. Temporal expression of several transcripts, including the transcription factors Nr4a1, Nr4a2, Egr1, Egr2, Btg1, and Btg2, and the epidermal growth factor (EGF)-like ligands Areg and Ereg, were analyzed by quantitative RT-PCR, and their putative roles in granulosa cell function are discussed. Epigen (Epgn), another member of the family of EGF-like ligands was identified for the first time in granulosa cells as rapidly induced by LH/hCG. We demonstrate that Epgn initiates cumulus expansion, similar to the other EGF-receptor ligands Areg and Ereg. -

Of the T Cell by G-CSF and IL-10 Cell Mobilization Requires Direct Signaling Modification of T Cell Responses by Stem
Downloaded from http://www.jimmunol.org/ by guest on September 29, 2021 is online at: average * The Journal of Immunology published online 28 February 2014 from submission to initial decision 4 weeks from acceptance to publication http://www.jimmunol.org/content/early/2014/02/28/jimmun ol.1302315 Modification of T Cell Responses by Stem Cell Mobilization Requires Direct Signaling of the T Cell by G-CSF and IL-10 Kelli P. A. MacDonald, Laetitia Le Texier, Ping Zhang, Helen Morris, Rachel D. Kuns, Katie E. Lineburg, Lucie Leveque, Alistair L. Don, Kate A. Markey, Slavica Vuckovic, Frederik O. Bagger, Glen M. Boyle, Bruce R. Blazar and Geoffrey R. Hill J Immunol Submit online. Every submission reviewed by practicing scientists ? is published twice each month by Receive free email-alerts when new articles cite this article. Sign up at: http://jimmunol.org/alerts http://jimmunol.org/subscription Submit copyright permission requests at: http://www.aai.org/About/Publications/JI/copyright.html http://www.jimmunol.org/content/suppl/2014/02/28/jimmunol.130231 5.DCSupplemental Information about subscribing to The JI No Triage! Fast Publication! Rapid Reviews! 30 days* Why • • • Material Permissions Email Alerts Subscription Supplementary The Journal of Immunology The American Association of Immunologists, Inc., 1451 Rockville Pike, Suite 650, Rockville, MD 20852 Copyright © 2014 by The American Association of Immunologists, Inc. All rights reserved. Print ISSN: 0022-1767 Online ISSN: 1550-6606. This information is current as of September 29, 2021. Published February 28, 2014, doi:10.4049/jimmunol.1302315 The Journal of Immunology Modification of T Cell Responses by Stem Cell Mobilization Requires Direct Signaling of the T Cell by G-CSF and IL-10 Kelli P. -

The Molecular Mechanisms by Which Vitamin D Prevents Insulin Resistance and Associated Disorders
International Journal of Molecular Sciences Review The Molecular Mechanisms by Which Vitamin D Prevents Insulin Resistance and Associated Disorders Izabela Szymczak-Pajor 1 ,Józef Drzewoski 2 and Agnieszka Sliwi´ ´nska 1,* 1 Department of Nucleic Acid Biochemistry, Medical University of Lodz, 251 Pomorska Str., 92-213 Lodz, Poland; [email protected] 2 Central Teaching Hospital of the Medical University of Lodz, 251 Pomorska Str., 92-213 Lodz, Poland; [email protected] * Correspondence: [email protected] Received: 9 August 2020; Accepted: 9 September 2020; Published: 11 September 2020 Abstract: Numerous studies have shown that vitamin D deficiency is very common in modern societies and is perceived as an important risk factor in the development of insulin resistance and related diseases such as obesity and type 2 diabetes (T2DM). While it is generally accepted that vitamin D is a regulator of bone homeostasis, its ability to counteract insulin resistance is subject to debate. The goal of this communication is to review the molecular mechanism by which vitamin D reduces insulin resistance and related complications. The university library, PUBMED, and Google Scholar were searched to find relevant studies to be summarized in this review article. Insulin resistance is accompanied by chronic hyperglycaemia and inflammation. Recent studies have shown that vitamin D exhibits indirect antioxidative properties and participates in the maintenance of normal resting ROS level. Appealingly, vitamin D reduces inflammation and regulates Ca2+ level in many cell types. Therefore, the beneficial actions of vitamin D include diminished insulin resistance which is observed as an improvement of glucose and lipid metabolism in insulin-sensitive tissues. -
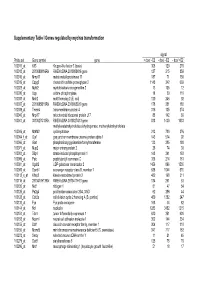
Supplementary Table I Genes Regulated by Myc/Ras Transformation
Supplementary Table I Genes regulated by myc/ras transformation signal Probe set Gene symbol gene + dox/ - E2 - dox/ - E2 - dox/ +E2 100011_at Klf3 Kruppel-like factor 3 (basic) 308 120 270 100013_at 2010008K16Rik RIKEN cDNA 2010008K16 gene 127 215 859 100016_at Mmp11 matrix metalloproteinase 11 187 71 150 100019_at Cspg2 chondroitin sulfate proteoglycan 2 1148 342 669 100023_at Mybl2 myeloblastosis oncogene-like 2 13 105 12 100030_at Upp uridine phosphorylase 18 39 110 100033_at Msh2 mutS homolog 2 (E. coli) 120 246 92 100037_at 2310005B10Rik RIKEN cDNA 2310005B10 gene 178 351 188 100039_at Tmem4 transmembrane protein 4 316 135 274 100040_at Mrpl17 mitochondrial ribosomal protein L17 88 142 89 100041_at 3010027G13Rik RIKEN cDNA 3010027G13 gene 818 1430 1033 methylenetetrahydrofolate dehydrogenase, methenyltetrahydrofolate 100046_at Mthfd2 cyclohydrolase 213 720 376 100064_f_at Gja1 gap junction membrane channel protein alpha 1 143 574 92 100066_at Gart phosphoribosylglycinamide formyltransferase 133 385 180 100071_at Mup2 major urinary protein 2 26 74 30 100081_at Stip1 stress-induced phosphoprotein 1 148 341 163 100089_at Ppic peptidylprolyl isomerase C 358 214 181 100091_at Ugalt2 UDP-galactose translocator 2 1456 896 1530 100095_at Scarb1 scavenger receptor class B, member 1 638 1044 570 100113_s_at Kifap3 kinesin-associated protein 3 482 168 311 100116_at 2810417H13Rik RIKEN cDNA 2810417H13 gene 134 261 53 100120_at Nid1 nidogen 1 81 47 54 100125_at Pa2g4 proliferation-associated 2G4, 38kD 62 286 44 100128_at Cdc2a cell division cycle 2 homolog A (S. pombe) 459 1382 247 100133_at Fyn Fyn proto-oncogene 168 44 82 100144_at Ncl nucleolin 1283 3452 1215 100151_at Tde1 tumor differentially expressed 1 620 351 620 100153_at Ncam1 neural cell adhesion molecule 1 302 144 234 100155_at Ddr1 discoidin domain receptor family, member 1 304 117 310 100156_at Mcmd5 mini chromosome maintenance deficient 5 (S. -

NF-Κb Signaling in Gastric Cancer
Review NF‐κB Signaling in Gastric Cancer Olga Sokolova and Michael Naumann * Institute of Experimental Internal Medicine, Otto von Guericke University Magdeburg, Magdeburg 39120, Germany; [email protected] * Correspondence: [email protected]; Tel.: +49‐391‐671‐3227 Abstract: Gastric cancer is a leading cause of cancer death worldwide. Diet, obesity, smoking and chronic infections, especially with Helicobacter pylori, contribute to stomach cancer development. H. pylori possesses a variety of virulence factors including encoded factors from the cytotoxin‐associated gene pathogenicity island (cagPAI) or vacuolating cytotoxin A (VacA). Most of the cagPAI‐encoded products form a type 4 secretion system (T4SS), a pilus‐like macromolecular transporter, which translocates CagA into the cytoplasm of the host cell. Only H. pylori strains carrying the cagPAI induce the transcription factor NF‐κB, but CagA and VacA are dispensable for direct NF‐κB activation. NF‐κB‐driven gene products include cytokines/chemokines, growth factors, anti‐apoptotic factors, angiogenesis regulators and metalloproteinases. Many of the genes transcribed by NF‐κB promote gastric carcinogenesis. Since it has been shown that chemotherapy‐caused cellular stress could elicit activation of the survival factor NF‐κB, which leads to acquisition of chemoresistance, the NF‐κB system is recommended for therapeutic targeting. Research is motivated for further search of predisposing conditions, diagnostic markers and efficient drugs to improve significantly the overall survival of patients. In this review, we provide an overview about mechanisms and consequences of NF‐κB activation in gastric mucosa in order to understand the role of NF‐κB in gastric carcinogenesis. Keywords: NF‐κB; inflammation; gastric cancer; Helicobacter pylori; VacA toxin; Type 4 secretion system; tumor microenvironment; chemoresistance 1. -

NR4A2 Is Regulated by Gastrin and Influences Cellular Responses of Gastric Adenocarcinoma Cells
NR4A2 Is Regulated by Gastrin and Influences Cellular Responses of Gastric Adenocarcinoma Cells Kristine Misund1, Linn-Karina Myrland Selvik1,2, Shalini Rao1,2, Kristin Nørsett1, Ingunn Bakke1, Arne K. Sandvik1,3, Astrid Lægreid1, Torunn Bruland1, Wenche S. Prestvik2, Liv Thommesen1,2* 1 Department of Cancer Research and Molecular Medicine, Norwegian University of Science and Technology (NTNU), Trondheim, Norway, 2 Faculty of Technology, Sør-Trøndelag University College, Trondheim, Norway, 3 Department of Gastroenterology and Hepatology, Medical Clinic, St. Olav’s University Hospital, Trondheim, Norway Abstract The peptide hormone gastrin is known to play a role in differentiation, growth and apoptosis of cells in the gastric mucosa. In this study we demonstrate that gastrin induces Nuclear Receptor 4A2 (NR4A2) expression in the adenocarcinoma cell lines AR42J and AGS-GR, which both possess the gastrin/CCK2 receptor. In vivo, NR4A2 is strongly expressed in the gastrin responsive neuroendocrine ECL cells in normal mucosa, whereas gastric adenocarcinoma tissue reveals a more diffuse and variable expression in tumor cells. We show that NR4A2 is a primary early transient gastrin induced gene in adenocarcinoma cell lines, and that NR4A2 expression is negatively regulated by inducible cAMP early repressor (ICER) and zinc finger protein 36, C3H1 type-like 1 (Zfp36l1), suggesting that these gastrin regulated proteins exert a negative feedback control of NR4A2 activated responses. FRAP analyses indicate that gastrin also modifies the nucleus-cytosol shuttling of NR4A2, with more NR4A2 localized to cytoplasm upon gastrin treatment. Knock-down experiments with siRNA targeting NR4A2 increase migration of gastrin treated adenocarcinoma AGS-GR cells, while ectopically expressed NR4A2 increases apoptosis and hampers gastrin induced invasion, indicating a tumor suppressor function of NR4A2. -

2004 Eastern Society for Pediatric Research 16Th Annual Meeting
2004 Eastern Society for Pediatric Research 16th Annual Meeting Program Guide March 26–28, 2004 Hyatt Regency Old Greenwich, CT In cooperation with the New York Academy of Medicine New for 2004 We are pleased to announce that we have engaged the services of the New York Academy of Medicine (NYAM) to run our 2004 meeting. They will also sponsor the CME program. While our recent meetings have been terrific, the administrative burden has exceeded what could reasonably be expected of our volunteers. We expect the engagement of the professional meeting planners at the NYAM will further enhance our meeting. This will include improved informatics enabling presenters to load their PowerPoint presentations at a central station in advance, avoiding some of the glitches we encountered last year. ESPR Officers and Council Sponsorship Honor Roll 2003–2004 President The ESPR expresses its appreciation to all of our Luc P. Brion, M.D. sponsors of the 2004 ESPR Annual Meeting Albert Einstein College of Medicine and Children’s Hospital at Montefiore, Established Sponsors Bronx, New York Curative Pharmacy Services Mead-Johnson Nutritionals Secretary-Treasurer Rashmin C. Savani, M.B.Ch.B. MedImmune, Inc. The University of Pennsylvania School of Medicine, Philips Medical Systems Philadelphia, Pennsylvania Ross Products Division of Abbott Laboratories, Inc. Chairperson, Planning Committee Additional Sponsors Bruce D. Gelb, M.D. Mount Sinai School of Medicine Dey L. P. New York, New York INO Therapeutics, Inc. Council Members Immediate Past President Mercury Medical Anthony Alario, MD Viasys Healthcare Mitchell J. Kresch, MD Clifford Bogue, MD Bruce D. Gelb, MD Academic Sponsors Ian Holzman, MD Past Presidents Alan R. -

Role of Nurr1 in Carcinogenesis and Tumor Immunology: a State of the Art Review
cancers Review Role of Nurr1 in Carcinogenesis and Tumor Immunology: A State of the Art Review Peter Kok-Ting Wan , Michelle Kwan-Yee Siu, Thomas Ho-Yin Leung, Xue-Tang Mo, Karen Kar-Loen Chan and Hextan Yuen-Sheung Ngan * Department of Obstetrics and Gynaecology, LKS Faculty of Medicine, the University of Hong Kong, Pok Fu Lam, Hong Kong, China; [email protected] (P.K.-T.W.); [email protected] (M.K.-Y.S.); [email protected] (T.H.-Y.L.); [email protected] (X.-T.M.); [email protected] (K.K.-L.C.) * Correspondence: [email protected]; Tel.: +852-2255-4260; Fax: +852-2855-0947 Received: 26 August 2020; Accepted: 14 October 2020; Published: 19 October 2020 Simple Summary: Nuclear receptor related-1 protein (Nurr1) emerges as a therapeutic target in multiple malignancies and immunotherapies. Previous studies have highlighted its association with clinicopathological parameters, tumorigenesis and therapeutic resistance in cancers. In addition, recent studies unraveled its contribution to the suppression of antitumor immunity, suggesting that inhibition of Nurr1 is a potential method to repress cancer aggressiveness and disrupt tumor immune tolerance. In line with this evidence, the present review provides the roles of Nurr1 in tumor progression and the associated underlying molecular mechanisms. Moreover, the significance of Nurr1 in promoting immune tolerance and potential strategies for Nurr1 inhibition are highlighted. Abstract: Nuclear receptor related-1 protein (Nurr1), coded by an early response gene, is involved in multiple cellular and physiological functions, including proliferation, survival, and self-renewal. Dysregulation of Nurr1 has been frequently observed in many cancers and is attributed to multiple transcriptional and post-transcriptional mechanisms. -

PTH and Vitamin D
PTH and Vitamin D Syed Jalal Khundmiri,1,2 Rebecca D. Murray,1,2 and Eleanor Lederer*1,2,3 ABSTRACT PTH and Vitamin D are two major regulators of mineral metabolism. They play critical roles in the maintenance of calcium and phosphate homeostasis as well as the development and mainte- nance of bone health. PTH and Vitamin D form a tightly controlled feedback cycle, PTH being a major stimulator of vitamin D synthesis in the kidney while vitamin D exerts negative feedback on PTH secretion. The major function of PTH and major physiologic regulator is circulating ionized calcium. The effects of PTH on gut, kidney, and bone serve to maintain serum calcium within a tight range. PTH has a reciprocal effect on phosphate metabolism. In contrast, vitamin D has a stimulatory effect on both calcium and phosphate homeostasis, playing a key role in providing adequate mineral for normal bone formation. Both hormones act in concert with the more recently discovered FGF23 and klotho, hormones involved predominantly in phosphate metabolism, which also participate in this closely knit feedback circuit. Of great interest are recent studies demon- strating effects of both PTH and vitamin D on the cardiovascular system. Hyperparathyroidism and vitamin D deficiency have been implicated in a variety of cardiovascular disorders including hypertension, atherosclerosis, vascular calcification, and kidney failure. Both hormones have di- rect effects on the endothelium, heart, and other vascular structures. How these effects of PTH and vitamin D interface with the regulation of bone formation are the subject of intense investigation. Published 2016. Compr Physiol 6:561-601, 2016.