Of Saxifraga Oppositifolia (Saxifragaceae)
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Rare Vascular Plant Surveys in the Polletts Cove and Lahave River Areas of Nova Scotia
Rare Vascular Plant Surveys in the Polletts Cove and LaHave River areas of Nova Scotia David Mazerolle, Sean Blaney and Alain Belliveau Atlantic Canada Conservation Data Centre November 2014 ACKNOWLEDGEMENTS This project was funded by the Nova Scotia Department of Natural Resources, through their Species at Risk Conservation Fund. The Atlantic Canada Conservation Data Centre appreciates the opportunity provided by the fund to have visited these botanically significant areas. We also thank Sean Basquill for mapping, fieldwork and good company on our Polletts Cove trip, and Cape Breton Highlands National Park for assistance with vehicle transportation at the start of that trip. PHOTOGRAPHY CREDITS All photographs included in this report were taken by the authors. 1 INTRODUCTION This project, funded by the Nova Scotia Species at Risk Conservation Fund, focused on two areas of high potential for rare plant occurrence: 1) the Polletts Cove and Blair River system in northern Cape Breton, covered over eight AC CDC botanist field days; and 2) the lower, non-tidal 29 km and selected tidal portions of the LaHave River in Lunenburg County, covered over 12 AC CDC botanist field days. The Cape Breton Highlands support a diverse array of provincially rare plants, many with Arctic or western affinity, on cliffs, river shores, and mature deciduous forests in the deep ravines (especially those with more calcareous bedrock and/or soil) and on the peatlands and barrens of the highland plateau. Recent AC CDC fieldwork on Lockhart Brook, Big Southwest Brook and the North Aspy River sites similar to the Polletts Cove and Blair River valley was very successful, documenting 477 records of 52 provincially rare plant species in only five days of fieldwork. -

Swing Through
Swing Through 20m Swing Through is an interactive agility garden that connects the user to Canada’s diverse landscape, as well as its major economic industry. The garden is a series of thirteen finished lumber posts that dangle from a large steel structure, creating “tree swings”. On the swings are climbing holds where visitors can use the holds to climb up and across the tree swings. Directly under the tree swings are thirteen colour-coordinated stumps that give the user an extra boost, if needed. The thirteen timber tree swings represent Canada’s ten provinces and three territories by using wood from the official provincial and territorial trees. Surrounding this structure of Canadian trees is a garden divided into thirteen sections displaying the native plants of each province and territory. This representative regional plantings encompassing the swings, creating a soft edge. 10m Swing Through allows visitors to touch, smell, and play with the various YT NT NU BC AB SK MB ON QC NL NB PE NS natural elements that make our country so green, prosperous and beautiful. PLAN | 1:75 Yukon Nunavut Alberta Manitoba Quebec New Brunswick Nova Scotia Tree: Subapline fir, Abies lasiocarpa Tree: Balsam Poplar, Populus balsamifera Tree: Lodgepole pine, Pinus contorta Tree: Balsam fir, Abies balsamea Tree: Yellow birch, Betula alleghaniensis Tree: Balsam fir, Abies balsamea Tree: Red spruce, Picea rubens Plants: Epilobium angustifolium, Plants: Saxifraga oppositifolia, Rubus Plants: Rosa acicularis Prunus virginiana, Plants: Pulsatilla ludoviciana, -
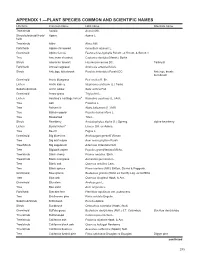
APPENDIX 1.—PLANT SPECIES COMMON and SCIENTIFIC NAMES Life Form Common Name Latin Name Alternate Name Tree/Shrub Acacia Acacia Mill
APPENDIX 1.—PLANT SPECIES COMMON AND SCIENTIFIC NAMES Life form Common name Latin name Alternate name Tree/shrub Acacia Acacia Mill. Shrub/subshrub//Forb/ Agave Agave L. herb Tree/shrub Alder Alnus Mill. Forb/herb Alpine chickweed Cerastium alpinum L. Graminoid Alpine fescue Festuca brachyphylla Schult. ex Schult. & Schult. f. Tree American chestnut Castanea dentata (Marsh.) Borkh. Shrub American tarwort Flourensia cernua DC. Tarbrush Forb/herb Annual ragweed Ambrosia artemisiifolia L. Shrub Antelope bitterbrush Purshia tridentata (Pursh) DC. Antelope brush; buckbrush Graminoid Arctic bluegrass Poa arctica R. Br. Lichen Arctic kidney Nephroma arcticum (L.) Torss. Subshrub/shrub Arctic willow Salix arctica Pall. Graminoid Arrow grass Triglochin L. Lichen Asahina’s cartilage lichend Ramalina asahinae (L.) Ach. Tree Ash Fraxinus L. Tree Balsam fi r Abies balsamea (L.) Mill. Tree Balsam poplar Populus balsamifera L. Tree Basswood Tilia L. Shrub Bearberry Arctostaphylos alpina (L.) Spreng. alpine bearberry Lichen Beard lichena Usnea Dill. ex Adans. Tree Beech Fagus L. Graminoid Big bluestem Andropogon gerardii Vitman Tree Big leaf maple Acer macrophyllum Pursh Tree/Shrub Big sagebrush Artemisia tridentata Nutt. Tree Bigtooth aspen Populus grandidentata Michx. Tree/shrub Black cherry Prunus serotina, Ehrh. Tree/shrub Black mangrove Avicennia germinans L. Tree Black oak Quercus velutina, Lam. Tree Black spruce Picea mariana (Mill.) Britton, Sterns & Poggenb. Graminoid Blue grama Bouteloua gracilis (Willd. ex Kunth) Lag. ex Griffi ths Tree Blue oak Quercus douglasii Hook. & Arn. Graminoid Bluestem Andropogon L. Tree Box elder Acer negundo L. Forb/herb Bracken fern Pteridium aquilinum var. pubescens Tree Bristlecone pine Pinus aristata Engelm. Subshrub/Shrub Brittlebush Encelia Adans. -

December 2014 ---International Rock Gardener--- December 2014
International Rock Gardener ISSN 2053-7557 Number 60 The Scottish Rock Garden Club December 2014 ---International Rock Gardener--- December 2014 The IRG Team sends our very best wishes and thanks to all readers and contributors around the world as we come to the close of 2014. This issue of IRG is our 60th – quite a milestone for what was begun as a one year experimental project. Production of the magazine does keep the IRG Team busy but of course, none of this would be possible without the generous input of our authors and photographers: To all of you - a special greeting of warm appreciation from Margaret, ZZ, Ian, Glassford and Richard. “Don’t tell me - more last minute editorial changes” from Theresa McCracken in Oregon So far, winter in the Northern Hemisphere has been veering between cold and mild to a degree that will confuse most of our plants, while in the South a drought is expected which will lead to problems for growers there. These are, I suppose, problems which are visited upon us most years, in some way or another. Perhaps this struggle against nature’s vagaries is part of what drives us in our challenge to “conquer” the difficulties and the puzzles our gardens present to us? Or perhaps we are just all happily obsessed? Cover picture: Dryas octopetala, the plant which is the SRGC’s emblem, from a painting by Anne Chambers www.srgc.net ISSN 2053-7557 ---International Rock Gardener--- ---Plant Portrait--- A Story of Saxifraga dinnikii forma alba by Frank Schmidt, photos the author, Adrian Young and Franto Paznocht Last autumn I was asked, at the Czech-German Meeting in Feuchtwangen, to write for the IRG about the history of Saxifraga dinnikii forma alba. -

Seeds of Success Program (SOS) Has Been Collecting Native Plant Seeds in Alaska for Over a Decade
Summary of Alaska Collections 2002-2012 AK025, AK040, AK930 A report submitted to BLM Alaska State Office 222 West 7th Avenue Anchorage AK 99501 Prepared by Alaska Natural Heritage Program University of Alaska Anchorage 707 A Street Anchorage AK 99501 and Michael Duffy Biological Consulting Services PO Box 243364 Anchorage AK 99524 Contents Introduction ……………………………………………………………… 1 Summary of collections …………………………………………………. 3 Seed storage and increase ………………………………………………… 5 Target list update ………………………………………………………… 8 Development of preliminary seed zones ………………………………… 12 Summary of collections by seed zone Arctic Alaska Seed Zone ………………………………………… 16 Interior Seed Zone ……………………………………………….. 20 West Alaska Seed Zone ………………………………………….. 26 Southwest Alaska Seed Zone …………………………………….. 32 South Central Alaska Seed Zone …………………………………. 34 Southeast Alaska Seed Zone ……………………………………… 40 Further recommendations ………………………………………………… 44 Literature cited …………………………………………………………… 45 Appendices ………………………………………………………………… 47 INTRODUCTION The Bureau of Land Management Seeds of Success Program (SOS) has been collecting native plant seeds in Alaska for over a decade. Beginning in 2002, collections have been made by staff from three offices: the Northern Field Office (whose SOS abbreviation is AK025), the Anchorage Field Office (AK040), and the Alaska State Office (AK930). Most of the AK025 and AK040 collections were made in partnership with the Kew Millennium Seed Bank Project (http://www.kew.org/science-conservation/save-seed- prosper/millennium-seed-bank/index.htm). Collecting trips over the period 2002-2007 produced 108 collections, and were made with the assistance of contract botanists from University of Alaska and the Alaska Plant Materials Center. With the conclusion of the Millennium Seed Bank partnership, the state program has focused on obtaining native plant seed to be stored and increased, with the objective of providing greater seed availability for restoration efforts. -

Jan S U D a Citační Ohlasy
Jan Suda Podklady ke jmenovacímu řízení – Citační ohlasy JAN S U D A CITAČNÍ OHLASY Citační ohlasy (s vyloučením autocitací) podle Web of Science k 1. 5. 2013 Celkový počet: 818 (včetně semiautocitací) / 670 (bez semiautocitací) (semiautocitace vyznačeny kurzivou) ----------------------------------------------------------------------------------------------------------------- Title: Estimation of nuclear DNA content in plants using flow cytometry Authors: Dolezel, J; Greilhuber, J; Suda, J Source: NATURE PROTOCOLS Volume: 2 Issue: 9 Pages: 2233-2244 DOI: 10.1038/nprot.2007.310 Published: 2007 1. Title: Role of adaptive and non-adaptive mechanisms forming complex patterns of genome size variation in six cytotypes of polyploid Allium oleraceum (Amaryllidaceae) on a continental scale Author(s): Duchoslav, Martin; Safarova, Lenka; Jandova, Michaela Source: ANNALS OF BOTANY Volume: 111 Issue: 3 Pages: 419-431 DOI: 10.1093/aob/mcs297 Published: MAR 2013 2. Title: Cellular aggregation is a key parameter associated with long term variability in paclitaxel accumulation in Taxus suspension cultures Author(s): Patil, Rohan A.; Kolewe, Martin E.; Roberts, Susan C. Source: PLANT CELL TISSUE AND ORGAN CULTURE Volume: 112 Issue: 3 Pages: 303-310 DOI: 10.1007/s11240-012-0237-3 Published: MAR 2013 3. Title: Chromosome counts and genome size of Leontopodium species (Asteraceae: Gnaphalieae) from south-western China Author(s): Russell, Anton; Safer, Stefan; Weiss-Schneeweiss, Hanna; et al. Source: BOTANICAL JOURNAL OF THE LINNEAN SOCIETY Volume: 171 Issue: 3 Pages: 627-636 DOI: 10.1111/boj.12011 Published: MAR 2013 4. Title: Nuclear genome size estimation and karyotype analysis of Lycium species (Solanaceae) Author(s): Chen, Jianjun; Liu, Xiaoli; Zhu, Linyao; et al. Source: SCIENTIA HORTICULTURAE Volume: 151 Pages: 46-50 DOI: 10.1016/j.scienta.2012.12.004 Published: FEB 28 2013 5. -

Vascular Flora and Geoecology of Mont De La Table, Gaspésie, Québec
RHODORA, Vol. 117, No. 969, pp. 1–40, 2015 E Copyright 2015 by the New England Botanical Club doi: 10.3119/14-07; first published on-line March 11, 2015. VASCULAR FLORA AND GEOECOLOGY OF MONT DE LA TABLE, GASPE´ SIE, QUE´ BEC SCOTT W. BAILEY USDA Forest Service, 234 Mirror Lake Road, North Woodstock, NH 03262 e-mail: [email protected] JOANN HOY 21 Steam Mill Road, Auburn, NH 03032 CHARLES V. COGBILL 82 Walker Lane, Plainfield, VT 05667 ABSTRACT. The influence of substrate lithology on the distribution of many vascular and nonvascular plants has long been recognized, especially in alpine, subalpine, and other rocky habitats. In particular, plants have been classified as dependent on high-calcium substrates (i.e., calcicoles) based on common restriction to habitats developed in calcareous rocks, such as limestone and marble. In a classic 1907 paper on the influence of substrate on plants, M. L. Fernald singled out a particular meadow on Mont de la Table in the Chic-Choc Mountains of Que´bec for its unusual co-occurrence of strict calcicole and calcifuge (i.e., acidophile) plant taxa. We re-located this site, investigated substrate factors responsible for its unusual plant diversity, and documented current plant distributions. No calcareous rocks were found on site. However, inclusions of calcareous rocks were found farther up the mountain. The highest pH and dissolved calcium concentrations in surface waters were found in a series of springs that deliver groundwater, presumably influenced by calcareous rocks up the slope. Within the habitat delineated by common occurrences of calcicole species, available soil calcium varied by a factor of five and soil pH varied by almost 1.5 units, depending on microtopography and relative connection with groundwater. -

Arctic Biodiversity Assessment
310 Arctic Biodiversity Assessment Purple saxifrage Saxifraga oppositifolia is a very common plant in poorly vegetated areas all over the high Arctic. It even grows on Kaffeklubben Island in N Greenland, at 83°40’ N, the most northerly plant locality in the world. It is one of the first plants to flower in spring and serves as the territorial flower of Nunavut in Canada. Zackenberg 2003. Photo: Erik Thomsen. 311 Chapter 9 Plants Lead Authors Fred J.A. Daniëls, Lynn J. Gillespie and Michel Poulin Contributing Authors Olga M. Afonina, Inger Greve Alsos, Mora Aronsson, Helga Bültmann, Stefanie Ickert-Bond, Nadya A. Konstantinova, Connie Lovejoy, Henry Väre and Kristine Bakke Westergaard Contents Summary ..............................................................312 9.4. Algae ..............................................................339 9.1. Introduction ......................................................313 9.4.1. Major algal groups ..........................................341 9.4.2. Arctic algal taxonomic diversity and regionality ..............342 9.2. Vascular plants ....................................................314 9.4.2.1. Russia ...............................................343 9.2.1. Taxonomic categories and species groups ....................314 9.4.2.2. Svalbard ............................................344 9.2.2. The Arctic territory and its subdivision .......................315 9.4.2.3. Greenland ...........................................344 9.2.3. The flora of the Arctic ........................................316 -

Annual Report 2016/17
ANNUAL REPORT 2016/17 OUR MISSION To be the authoritative, primary source of accessible, current, and reliable information on the distribution and abundance of Canada’s natural diversity—especially species and ecosystems of conservation concern. From the Chair and Executive Director Table of Contents nother busy and very successful year for NatureServe Canada (NSC) and its 2 From the Chair and Executive Director membership! We were pleased to welcome Fisheries and Oceans Canada as a new Associate Member. Our membership now includes all three responsible federal 3 News A departments under Canada’s Species at Risk Act. This is a significant step forward in our 4 Connecting Science with Conservation efforts to improve communications and foster collaboration between organizations mandated to develop knowledge and achieve conservation outcomes for Canada’s rare and threatened 5 Who We Are/What We Do species. 6 Benefits to Conservation This past year NSC developed and released our latest report On Guard For Them: Species of NatureServe Canada Network in Action Global Conservation Concern in Canada. Working with our membership and partners, NSC assessed the global ranks of over 7000 species, subspecies and plant varieties. Released at an Bioblitzing A New Nature Reserve in Ontario 7 evening gala at the international NatureServe Biodiversity Without Boundaries conference in 8 Tracking a Rare Salamander in Manitoba April 2017, the report garnered a wave of media attention including features by the Globe and Mail, CBC radio and television, Huffington Post, and Yahoo. Our message regarding the status 9 Rediscovering Historical Species in Yukon of Canada’s biodiversity and the scientific knowledge developed and distributed by the NSC Network greatly increased its reach, informing a broad audience in Canada and beyond. -

Burnt Cape Ecological Reserve Management Plan
MANAGEMENT PLAN BURNT CAPE ECOLOGICAL RESERVE Parks and Natural Areas Division Department of Environment and Conservation Government of Newfoundland and Labrador 2000 Table of Contents 1.0 INTRODUCTION .................................... ............ 1 2.0 BACKGROUND INFORMATION .......................... ........ 2 3.0 SITE DESCRIPTION................................ .............. 4 4.0 CLIMATE ......................................... ............. 5 5.0 GEOLOGY............................................ ...........7 6.0 FAUNA ........................................... ............. 8 7.0 FLORA........................................... ..............10 8.0 LAND USE ACTIVITIES ..................................... ..... 14 9.0 SUMMARY............................................ .............. 15 10.0 MANAGEMENT POLICIES ............................ ........... 15 REFERENCES ......................................... .............. 19 1.0 INTRODUCTION The Wilderness and Ecological Reserves Act (WER Act) 1980, provides for the protection of important natural areas in Newfoundland and Labrador. These areas are established for preservation of natural ecosystems, outdoor recreation pursuits, scientific research and public education. More specifically, the WER Act provides the following objectives for establishing ecological reserves: S to provide for scientific research and educational purposes in aspects of the natural environment; S to preserve the habitat of an animal or plant species that is rare or endangered; S to provide standards -

Plants of Eastern Newfoundland, TODD BOLAND 3
ROCK GARDEN Quarterly Volume 63 Number 1 Winter 2005 Front cover: Townsendia condensata. Painting by Cindy Nelson-Nold. Back cover: Alpines on Mount Sefton, New Zealand by Dick Redfield. All material copyright ©2005 North American Rock Garden Society Printed by Allen Press, 800 E. 10th St., Lawrence, Kansas ROCK GARDEN Quarterly BULLETIN OF THE NORTH AMERICAN ROCK GARDEN SOCIETY Volume 63 Number 1 Winter 2005 Contents Special Issue: North Atlantic Rock Gardening Ericaceous Plants of Eastern Newfoundland, TODD BOLAND 3 Arctic-AlpineWillows of Newfoundland and Labrador, MARIA GALLETTI 7 Southern Alpines in St. John's, BODIL LARSEN 11 Growing Small Bulbs in Newfoundland, HOWARD CLASE 15 Saxifrages of Newfoundland and Labrador, TODD BOLAND 37 A New Atlantic Rock Garden, BERNARD JACKSON 40 Arctic-Alpine Plants of Western Greenland, TODD BOLAND 43 Rock Gardening at Wave Hill, LOLA LLOYD HORWITZ 46 The Art of Splitting Boulders, DAVID SELLARS 50 Why "Rock"?, NICHOLAS KLISE 53 PLANT PORTRAITS Townsendia condensata, RICK LUPP 56 Cyclamen purpurascens, GERALD R. FIRAK 56 Iris setosa subsp. canadensis, TODD BOLAND 58 BOOKS Halda, The Genus Paeonia, rev. by JOHN GRIMSHAW 60 States, Wild/lowers of Wyoming, rev. by RICHARD A. WEIGEL 62 Harlow & Jakob, Wild Lilies, Irises and Grasses, rev. by DIANA CHAPMAN 63 2004 Photo Contest Results 65 IN MEMOPJAM: Richard Redfield 68 Special Issue: Rock Gardens on the North Atlantic his winter issue focuses on the site of this summer's NARGS Annual General TMeeting, held in one of the Society's more remote outposts in St. John's, Newfoundland. Those who attend can look forward to seeing the picturesque coast and harbor towns of Canada's Atlantic coast, as well as its wild flora and public and private gardens. -

Sarraceniasarracenia Volume 17, Number 4
SarraceniaSarracenia Volume 17, Number 4. Winter 2010 ISSNs: 1920-5821 (Print) 1920-583X (Online) Newsletter of the Wildflower Society of Newfoundland and Labrador. C/o Botanical Garden, Memorial University, St John's, NL, A1C 5S7 e-mail: [email protected] Contents A Note from the Editor, Christmas Competition, Upcoming meetings...........................................................48 The Northern Peninsula in Spring by Leila Clase............................................................................................................................................48 Avalonia Field Trip Memories: Day Two. Monday July 20 2009 By Ed Hayden...........................................................................................................................................50 2009 Photo-competition: Second Place winners...........................................................................................55 Geranium robertianum & Rhizocarpon geographicum at Point Verde, (see p. 54) Judith Blakeley Sarracenia Vol. 17 #4 A Note from the Editor. from the wild. It recently came to my attention that so far there have Associated winter walk. Place time and date TBA, but th been only three issues in volume 17 (not counting the probably Sunday afternoon, February 7 . Christmas special) so this is a quick issue to wrap up the Wednesday March 3rd 2010 ,at 7.30 p.m* volume, and get out the volume index. I already have Todd Boland will give a talk and slideshow on the some material for Vol 18#1, but would welcome more. Orchids and other Wild Flowers of Equador. Christmas Competition. *At the Botanical Garden Lecture Room. Sadly there were only three entries, all correct apart The 2009-10 Executive President: Carmel Conway 722-0121 from one minor typo from: John Maunder, Mike Collins [email protected] and Stuart Hay. The prize was awarded to Stuart, as the Vice-President: John Maunder 335-2462 other two already had a copy of the book.