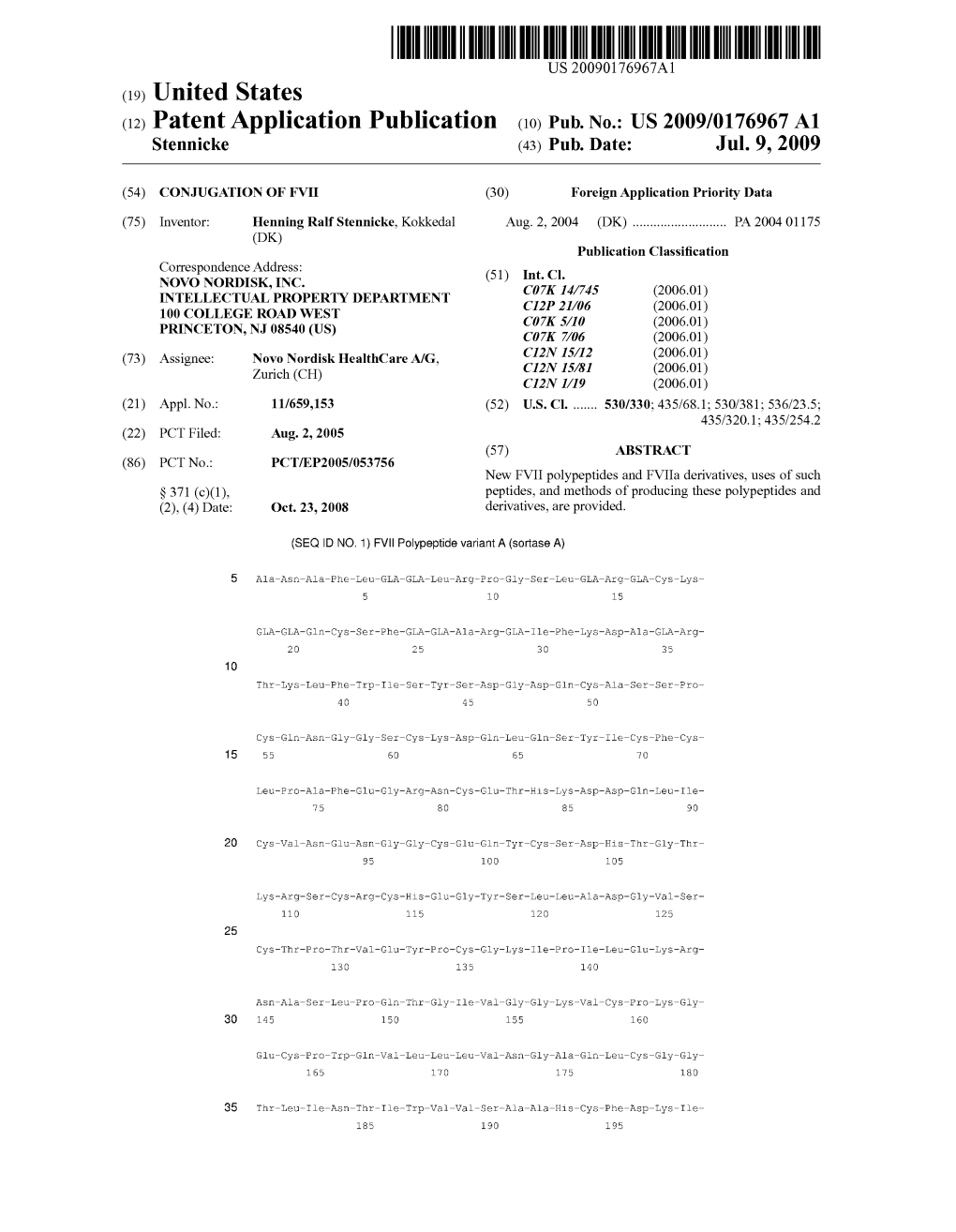
(12) Patent Application Publication (10) Pub. No.: US 2009/0176967 A1 Stennicke (43) Pub
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Serine Proteases with Altered Sensitivity to Activity-Modulating
(19) & (11) EP 2 045 321 A2 (12) EUROPEAN PATENT APPLICATION (43) Date of publication: (51) Int Cl.: 08.04.2009 Bulletin 2009/15 C12N 9/00 (2006.01) C12N 15/00 (2006.01) C12Q 1/37 (2006.01) (21) Application number: 09150549.5 (22) Date of filing: 26.05.2006 (84) Designated Contracting States: • Haupts, Ulrich AT BE BG CH CY CZ DE DK EE ES FI FR GB GR 51519 Odenthal (DE) HU IE IS IT LI LT LU LV MC NL PL PT RO SE SI • Coco, Wayne SK TR 50737 Köln (DE) •Tebbe, Jan (30) Priority: 27.05.2005 EP 05104543 50733 Köln (DE) • Votsmeier, Christian (62) Document number(s) of the earlier application(s) in 50259 Pulheim (DE) accordance with Art. 76 EPC: • Scheidig, Andreas 06763303.2 / 1 883 696 50823 Köln (DE) (71) Applicant: Direvo Biotech AG (74) Representative: von Kreisler Selting Werner 50829 Köln (DE) Patentanwälte P.O. Box 10 22 41 (72) Inventors: 50462 Köln (DE) • Koltermann, André 82057 Icking (DE) Remarks: • Kettling, Ulrich This application was filed on 14-01-2009 as a 81477 München (DE) divisional application to the application mentioned under INID code 62. (54) Serine proteases with altered sensitivity to activity-modulating substances (57) The present invention provides variants of ser- screening of the library in the presence of one or several ine proteases of the S1 class with altered sensitivity to activity-modulating substances, selection of variants with one or more activity-modulating substances. A method altered sensitivity to one or several activity-modulating for the generation of such proteases is disclosed, com- substances and isolation of those polynucleotide se- prising the provision of a protease library encoding poly- quences that encode for the selected variants. -

Anaerobic Bacteria Confirmed Plenary Speakers
OFFICIALOFFICIAL JOURNALJOURNAL OFOF THETHE AUSTRALIAN SOCIETY FOR MICROBIOLOGY INC.INC. VolumeVolume 3636 NumberNumber 33 SeptemberSeptember 20152015 Anaerobic bacteria Confirmed Plenary speakers Professor Peter Professor Dan Assoc Prof Susan Lynch Dr Brian Conlon Professor Anna Hawkey Andersson University of California Northeastern Durbin University of Upsalla University San Francisco University, Boston Johns Hopkins Birmingham Environmental pollution Colitis, Crohn's Disease Drug discovery in Dengue and vaccines Nosocomial by antibiotics and its and Microbiome soil bacteria infection control and role in the evolution of Research antibiotic resistance resistance As with previous years, ASM 2016 will be co-run with NOW CONFIRMED! EduCon 2016: Microbiology Educators’ Conference 2016 Rubbo Oration Watch this space for more details on the scientific and Professor Anne Kelso social program, speakers, ASM Public Lecture, workshops, CEO NHMRC ASM awards, student events, travel awards, abstract deadlines and much more.. Perth, WA A vibrant and beautiful city located on the banks of the majestic Swan river. Come stay with us in WA and experience our world class wineries and restaurants, stunning national parks, beaches and much more.. www.theasm.org.au www.westernaustralia.theasm.org.au Annual Scientific Meeting and Trade Exhibition The Australian Society for Microbiology Inc. OFFICIAL JOURNAL OF THE AUSTRALIAN SOCIETY FOR MICROBIOLOGY INC. 9/397 Smith Street Fitzroy, Vic. 3065 Tel: 1300 656 423 Volume 36 Number 3 September 2015 Fax: 03 9329 1777 Email: [email protected] www.theasm.org.au Contents ABN 24 065 463 274 Vertical For Microbiology Australia Transmission 102 correspondence, see address below. Jonathan Iredell Editorial team Guest Prof. Ian Macreadie, Mrs Jo Macreadie Editorial 103 and Mrs Hayley Macreadie Anaerobic bacteria 103 Editorial Board Dena Lyras and Julian I Rood Dr Chris Burke (Chair) Dr Gary Lum Under the Prof. -

Directed Sortase Evolution for Site-Specific Protein Engineering and Surface Functionalization
Directed sortase evolution for site-specific protein engineering and surface functionalization Von der Fakultät für Mathematik, Informatik und Naturwissenschaften der RWTH Aachen University zur Erlangung des akademischen Grades einer Doktorin der Naturwissenschaften genehmigte Dissertation vorgelegt von Zhi Zou Master of Biochemistry and Molecular Biology aus Huanggang, Hubei, P.R. China Berichter: Univ.-Prof. Dr. rer. nat. Ulrich Schwaneberg Univ.-Prof. Dr. rer. nat. Andrij Pich Tag der mündlichen Prüfung: 26.02.2019 Diese Dissertation ist auf den Internetseiten der Universitätsbibliothek verfügbar. Table of Contents Table of Contents Acknowledgements ....................................................................................................................................... 6 Abbreviations and acronyms ......................................................................................................................... 7 Abstract .......................................................................................................................................................... 9 1. Chapter I: Introduction ............................................................................................................................ 11 1.1. Sortases: sources, classes, and functions ......................................................................................... 11 1.1.1 Class A sortases: sortase A ........................................................................................................................ -

Modulation of Listeria Monocytogenes Biofilm Formation Using Small Molecules and Enzymes
MODULATION OF LISTERIA MONOCYTOGENES BIOFILM FORMATION USING SMALL MOLECULES AND ENZYMES MODULATION OF LISTERIA MONOCYTOGENES BIOFILM FORMATION USING SMALL MOLECULES AND ENZYMES By UYEN THI TO NGUYEN, B.Sc. A Thesis Submitted to the School of Graduate Studies in Partial Fulfillment of the Requirements for the Degree Doctor of Philosophy McMaster University © Copyright by Uyen T.T. Nguyen, July 2014 Ph.D. – U.T.T. Nguyen; McMaster University – Biochemistry and Biomedical Sciences McMaster University DOCTOR OF PHILOSOPHY (2014) Hamilton, Ontario (Biochemistry and Biomedical Sciences) TITLE: Modulation of Listeria monocytogenes biofilm formation using small molecules and enzymes AUTHOR: Uyen Thi To Nguyen, B.Sc. (McMaster University) SUPERVISOR: Dr. Lori L. Burrows NUMBER OF PAGES: xvii, 217 ii Ph.D. – U.T.T. Nguyen; McMaster University – Biochemistry and Biomedical Sciences ABSTRACT Inadequately disinfected food contact surfaces colonized by Listeria monocytogenes can come into contact with ready-to-eat food products causing cross-contamination and food-borne outbreaks. L. monocytogenes is tolerant of high salt, low temperatures and low pH, in part due to its ability to form biofilms, defined as communities of microorganisms that are surrounded by a self-produced extracellular polymeric substance that can adhere to surfaces. Biofilm formation is a complex process involving a series of poorly defined physiological changes that together lead to tolerance of disinfectants and antibiotics. To better understand the process of L. monocytogenes biofilm development, and to investigate ways in which colonization of surfaces might be prevented, we developed a microtiter biofilm assay suitable for high throughput screening. The assay was used to identify small molecules (protein kinase inhibitors and previously FDA-approved bioactive drugs) that modulate L. -

Genome Structure of the Symbiont Bifidobacterium
fmicb-07-00624 April 27, 2016 Time: 13:28 # 1 ORIGINAL RESEARCH published: 29 April 2016 doi: 10.3389/fmicb.2016.00624 Genome Structure of the Symbiont Bifidobacterium pseudocatenulatum CECT 7765 and Gene Expression Profiling in Response to Lactulose-Derived Oligosaccharides Alfonso Benítez-Páez1*, F. Javier Moreno2, María L. Sanz3 and Yolanda Sanz1 1 Microbial Ecology, Nutrition and Health Research Group, Instituto de Agroquímica y Tecnología de Alimentos – Consejo Superior de Investigaciones Científicas, Paterna, Spain, 2 Instituto de Investigación en Ciencias de la Alimentación, CIAL (CSIC-UAM), CEI (UAMCCSIC), Madrid, Spain, 3 Instituto de Química Orgánica General – Consejo Superior de Edited by: Investigaciones Científicas, Madrid, Spain M. Pilar Francino, FISABIO_Public Health, Valencian Health Department, Spain Bifidobacterium pseudocatenulatum CECT 7765 was isolated from stools of a breast- Reviewed by: fed infant. Although, this strain is generally considered an adult-type bifidobacterial Alberto Finamore, species, it has also been shown to have pre-clinical efficacy in obesity models. In Council for Agricultural Research and Economics–Food and Nutrition order to understand the molecular basis of its adaptation to complex carbohydrates Research Center, Italy and improve its potential functionality, we have analyzed its genome and transcriptome, Simone Rampelli, University of Bologna, Italy as well as its metabolic output when growing in galacto-oligosaccharides derived *Correspondence: from lactulose (GOS-Lu) as carbon source. B. pseudocatenulatum CECT 7765 shows Alfonso Benítez-Páez strain-specific genome regions, including a great diversity of sugar metabolic-related [email protected] genes. A preliminary and exploratory transcriptome analysis suggests candidate over- Specialty section: expression of several genes coding for sugar transporters and permeases; furthermore, This article was submitted to five out of seven beta-galactosidases identified in the genome could be activated in Microbial Symbioses, response to GOS-Lu exposure. -

Clostridium Difficile Has a Single Sortase, Srtb, That Can Be Inhibited by Small-Molecule Inhibitors
Donahue et al. BMC Microbiology 2014, 14:219 http://www.biomedcentral.com/1471-2180/14/219 RESEARCH ARTICLE Open Access Clostridium difficile has a single sortase, SrtB, that can be inhibited by small-molecule inhibitors Elizabeth H Donahue1, Lisa F Dawson1, Esmeralda Valiente1, Stuart Firth-Clark2, Meriel R Major2, Eddy Littler2, Trevor R Perrior2 and Brendan W Wren1* Abstract Background: Bacterial sortases are transpeptidases that covalently anchor surface proteins to the peptidoglycan of the Gram-positive cell wall. Sortase protein anchoring is mediated by a conserved cell wall sorting signal on the anchored protein, comprising of a C-terminal recognition sequence containing an “LPXTG-like” motif, followed by a hydrophobic domain and a positively charged tail. Results: We report that Clostridium difficile strain 630 encodes a single sortase (SrtB). A FRET-based assay was used to confirm that recombinant SrtB catalyzes the cleavage of fluorescently labelled peptides containing (S/P)PXTG motifs. Strain 630 encodes seven predicted cell wall proteins with the (S/P)PXTG sorting motif, four of which are conserved across all five C. difficile lineages and include potential adhesins and cell wall hydrolases. Replacement of the predicted catalytic cysteine residue at position 209 with alanine abolishes SrtB activity, as does addition of the cysteine protease inhibitor MTSET to the reaction. Mass spectrometry reveals the cleavage site to be between the threonine and glycine residues of the (S/P)PXTG peptide. Small-molecule inhibitors identified through an in silico screen inhibit SrtB enzymatic activity to a greater degree than MTSET. Conclusions: These results demonstrate for the first time that C. -

UNIVERSITY of CALIFORNIA Los Angeles
UNIVERSITY OF CALIFORNIA Los Angeles Elucidating the Molecular Basis of Protein and Polymer Display in Gram-Positive Bacteria for Novel Antibiotic Development A dissertation submitted in partial satisfaction of the requirements for the degree Doctor of Philosophy in Molecular Biology by Michele Diedre Kattke 2017 © Copyright by Michele Diedre Kattke 2017 ABSTRACT OF THE DISSERTATION Elucidating the Molecular Basis of Protein and Polymer Display in Gram-Positive Bacteria for Novel Antibiotic Development by Michele Diedre Kattke Doctor of Philosophy in Molecular Biology University of California, Los Angeles, 2017 Professor Robert Thompson Clubb, Chair The emergence of multi-drug resistant bacteria has prompted novel antibiotic development by targeting non-essential pathways, such as virulence factor production and display during cell wall biosynthesis. Within Gram-positive bacteria, sortase transpeptidases covalently attach proteins to the cell wall or assemble pili using class A-F enzymes. Interestingly, class E sortases display proteins via recognition of a non-canonical LAXTG motif. We have determined the first crystal structure of a class E sortase, the 1.93 Å resolution structure of SrtE1 from Streptomyces coelicolor. The SrtE1 enzyme possesses structurally distinct β3/β4 and β6/β7 active site loops that contact the LAXTG substrate. Furthermore, molecular dynamics studies have identified a conserved tyrosine residue that likely confers substrate specificity for class E sortases. A second anti-virulence target, the TarA glycosyltransferase (GT), is highly conserved among Gram-positive bacteria and produces surface-anchored wall teichoic acid (WTA) polymers. The WTA biosynthetic mechanism involving TarA and other membrane- associated, enzymes is poorly understood due to a lack of structural characterization. -

Sortase Enzymes and Their Integral Role in the Development of Streptomyces Coelicolor
Sortase enzymes and their integral role in the development of Streptomyces coelicolor Sortase enzymes and their integral role in the development of Streptomyces coelicolor Andrew Duong A Thesis Submitted to the School of Graduate Studies In Partial Fulfillment of the Requirements of the Degree of Master of Science McMaster University Copyright by Andrew Duong, December, 2014 Master of Science (2014) McMaster University (Biology) Hamilton, Ontario TITLE: Sortase enzymes and their integral role in the development of Streptomyces coelicolor AUTHOR: Andrew Duong, B.Sc. (H) (McMaster University) SUPERVISOR: Dr. Marie A. Elliot NUMBER OF PAGES: VII, 77 Abstract Sortase enzymes are cell wall-associated transpeptidases that facilitate the attachment of proteins to the peptidoglycan. Exclusive to Gram positive bacteria, sortase enzymes contribute to many processes, including virulence and pilus attachment, but their role in Streptomyces coelicolor biology remained elusive. Previous work suggested that the sortases anchored a subset of a group of hydrophobic proteins known as the long chaplins. The chaplins are important in aerial hyphae development, where they are secreted from the cells and coat the emerging aerial hyphae to reduce the surface tension at the air-aqueous interface. Two sortases (SrtE1 and SrtE2) were predicted to anchor these long chaplins to the cell wall of S. coelicolor. Deletion of both sortases or long chaplins revealed that although the long chaplins were dispensable for wild type-like aerial hyphae formation, the sortase mutant had a severe defect in growth. These two sortases were found to be nearly redundant, as deletion of individual enzymes led to only a modest change in phenotype. -

Thesis Submitted for the Degree of Doctor of Philosophy
University of Bath PHD Investigation of the Clostridium difficile Sortase by Gene Knockout, X-ray Crystallography and Biochemical Characterisation Chambers, Christopher Award date: 2014 Awarding institution: University of Bath Link to publication Alternative formats If you require this document in an alternative format, please contact: [email protected] General rights Copyright and moral rights for the publications made accessible in the public portal are retained by the authors and/or other copyright owners and it is a condition of accessing publications that users recognise and abide by the legal requirements associated with these rights. • Users may download and print one copy of any publication from the public portal for the purpose of private study or research. • You may not further distribute the material or use it for any profit-making activity or commercial gain • You may freely distribute the URL identifying the publication in the public portal ? Take down policy If you believe that this document breaches copyright please contact us providing details, and we will remove access to the work immediately and investigate your claim. Download date: 07. Oct. 2021 Investigation of the Clostridium difficile Sortase by Gene Knockout, X-ray Crystallography and Biochemical Characterisation Christopher James Chambers A Thesis Submitted for the Degree of Doctor of Philosophy University of Bath Department of Biology and Biochemistry December 2013 COPYRIGHT Attention is drawn to the fact that copyright of this thesis rests with its author. A copy of this thesis has been supplied on condition that anyone who consults it is understood to recognise that its copyright rests with the author and they must not copy it or use material from it except as permitted by law or with the consent of the author. -

First Characterization of a Class F Sortase and Establishment of a Microreactor-Based Assay for Its Directed Evolution
First Characterization of a Class F Sortase and Establishment of a Microreactor-Based Assay for its Directed Evolution Inauguraldissertation zur Erlangung der Würde eines Doktors der Philosophie vorgelegt der Philosophisch-Naturwissenschaftlichen Fakultät der Universität Basel von Salvatore Di Girolamo aus Italien 2020 Originaldokument gespeichert auf dem Dokumentenserver der Universität Basel edoc.unibas.ch Dieses Werk ist lizenziert unter einer Creative Commons Namensnennung 4.0 International Lizenz. Genehmigt von der Philosophisch-Naturwissenschaftlichen Fakultät der Universität Basel auf Antrag von Prof. Dr. Florian Seebeck Prof. Dr. Georg Lipps Prof. Dr. Michael Nash Basel, 21 Mai 2019 Prof. Dr. Martin Spiess 1 2 Table of contents ABSTRACT ........................................................................................................................................... 5 LIST OF ABBREVIATIONS ............................................................................................................ 6 1 INTRODUCTION ........................................................................................................................ 8 1.1 Biology and function of sortases ............................................................................................................ 9 1.2 Classification of sortases ..................................................................................................................... 10 1.3 Sortases structure and mechanism of action...................................................................................... -

All Enzymes in BRENDA™ the Comprehensive Enzyme Information System
All enzymes in BRENDA™ The Comprehensive Enzyme Information System http://www.brenda-enzymes.org/index.php4?page=information/all_enzymes.php4 1.1.1.1 alcohol dehydrogenase 1.1.1.B1 D-arabitol-phosphate dehydrogenase 1.1.1.2 alcohol dehydrogenase (NADP+) 1.1.1.B3 (S)-specific secondary alcohol dehydrogenase 1.1.1.3 homoserine dehydrogenase 1.1.1.B4 (R)-specific secondary alcohol dehydrogenase 1.1.1.4 (R,R)-butanediol dehydrogenase 1.1.1.5 acetoin dehydrogenase 1.1.1.B5 NADP-retinol dehydrogenase 1.1.1.6 glycerol dehydrogenase 1.1.1.7 propanediol-phosphate dehydrogenase 1.1.1.8 glycerol-3-phosphate dehydrogenase (NAD+) 1.1.1.9 D-xylulose reductase 1.1.1.10 L-xylulose reductase 1.1.1.11 D-arabinitol 4-dehydrogenase 1.1.1.12 L-arabinitol 4-dehydrogenase 1.1.1.13 L-arabinitol 2-dehydrogenase 1.1.1.14 L-iditol 2-dehydrogenase 1.1.1.15 D-iditol 2-dehydrogenase 1.1.1.16 galactitol 2-dehydrogenase 1.1.1.17 mannitol-1-phosphate 5-dehydrogenase 1.1.1.18 inositol 2-dehydrogenase 1.1.1.19 glucuronate reductase 1.1.1.20 glucuronolactone reductase 1.1.1.21 aldehyde reductase 1.1.1.22 UDP-glucose 6-dehydrogenase 1.1.1.23 histidinol dehydrogenase 1.1.1.24 quinate dehydrogenase 1.1.1.25 shikimate dehydrogenase 1.1.1.26 glyoxylate reductase 1.1.1.27 L-lactate dehydrogenase 1.1.1.28 D-lactate dehydrogenase 1.1.1.29 glycerate dehydrogenase 1.1.1.30 3-hydroxybutyrate dehydrogenase 1.1.1.31 3-hydroxyisobutyrate dehydrogenase 1.1.1.32 mevaldate reductase 1.1.1.33 mevaldate reductase (NADPH) 1.1.1.34 hydroxymethylglutaryl-CoA reductase (NADPH) 1.1.1.35 3-hydroxyacyl-CoA -

(12) United States Patent (10) Patent No.: US 8,993,295 B2 Seed Et Al
US008993295 B2 (12) United States Patent (10) Patent No.: US 8,993,295 B2 Seed et al. (45) Date of Patent: *Mar. 31, 2015 (54) METHODS, COMPOSITIONS, AND KITS FOR (56) References Cited THE SELECTIVE ACTIVATION OF PROTOXINS THROUGH COMBINATORIAL U.S. PATENT DOCUMENTS TARGETING 4,975,278 A 12/1990 Senter 5,156,840 A 10, 1992 Goers (75) Inventors: Brian Seed, Boston, MA (US); Jia Liu 6,258,360 B1 7/2001 Von Borstel 2002/0142359 A1 10/2002 Copley Wolfe, Winchester, MA (US); Glen S. 2003, OO54000 A1 3/2003 Dowdy Cho, Brookline, MA (US); Chia-Iun 2004/0048784 A1 3/2004 Keener et al. Tsai, Winchester, MA (US) 2009/00 16988 A1* 1/2009 Buckley ....................... 424/85.2 2010/0256070 A1* 10/2010 Seed et al. ................... 514, 19.3 (73) Assignee: The General Hospital Corporation, Boston, MA (US) FOREIGN PATENT DOCUMENTS WO WO98, 20135 A2 5, 1998 (*) Notice: Subject to any disclaimer, the term of this WO WOO1/14570 A1 3, 2001 patent is extended or adjusted under 35 WO WO 2004/094478 A2 11/2004 U.S.C. 154(b) by 1188 days. This patent is Subject to a terminal dis OTHER PUBLICATIONS claimer. Chiron et al. (JBC 272(50):31707-31711 (1997)).* Nygren et al., “Overview of the clinical efficacy of investigational (21) Appl. No.: 12/374,616 anticancer drugs” Journal of Internal Medicine. 253:46-75 (2003). Stenter et al., “Activation of prodrugs by antibody-enzyme conju (22) PCT Fled: Jul. 20, 2007 gates: a new approach to cancer therapy.” The FASEBJournal 4:188 193 (1990).