White-Chinned Petrel Distribution, Abundance and Connectivity: NZ Populations and Their Global Context
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

The Stormy Petrel (Procellaria Pelagica)
THE 0.8~ O.SEMI-ANNUAL. 37 THE STORMYPETREL. Procellaria Pelagica. DY W. RAINE, TORONTO, CANADA This interesting little bird, though rare in North America, is plen- tiful on the British side of the Atlantic Ocean. It is supposed to be the smallest web-footed bird known, and seldom comes to shore ex- cept during the breeding season, when they resort to such places as the Stilly Islands, in the English Channel, and the islands of the Irish Sea; but their chief nesting places are in the Orkney and Shetland Islands, and St. Kilda and STORMY PETREL. the outer Hebrides. This bird is well known to sailors by the name of Mother Carey’s Chicken, and hated by them because it foretells an approaching storm. They are mostly seen in stormy weather, because the marine crea- tures, on which they feed, are tossed to the surface of the chopping waves, and can be easily picked up by the bird as it passes over the waves, pattering the water with its webbed feet, and flapping its wings so as to keep itself just above the surface. The name Petrel is given to these birds on account of its powers of walking on the water, as is related of St. Peter. This bird seems very happy during rough weather, and many a ship-wrecked sailor, while clinging half locausted to some floating wreckage, has envied this little bird of its powers of flight, as it traverses the rolling, seething billows with wonderful ease. It feeds on the little fish, crustaceaus and molluses which are found in abundance on the surface of the sea. -

Cytochrome-B Evidence for Validity and Phylogenetic Relationships of Pseudobulweria and Bulweria (Procellariidae)
The Auk 115(1):188-195, 1998 CYTOCHROME-B EVIDENCE FOR VALIDITY AND PHYLOGENETIC RELATIONSHIPS OF PSEUDOBULWERIA AND BULWERIA (PROCELLARIIDAE) VINCENT BRETAGNOLLE,•'5 CAROLE A3VFII•,2 AND ERIC PASQUET3'4 •CEBC-CNRS, 79360 Beauvoirsur Niort, France; 2Villiers en Bois, 79360 Beauvoirsur Niort, France; 3Laboratoirede ZoologieMammi•res et Oiseaux,Museum National d'Histoire Naturelie, 55 rue Buffon,75005 Paris, France; and 4Laboratoirede Syst•matiquemol•culaire, CNRS-GDR 1005, Museum National d'Histoire Naturelie, 43 rue Cuvier, 75005 Paris, France ABSTRACT.--Althoughthe genus Pseudobulweria was described in 1936for the Fiji Petrel (Ps.macgillivrayi), itsvalidity, phylogenetic relationships, and the number of constituenttaxa it containsremain controversial. We tried to clarifythese issues with 496bp sequencesfrom the mitochondrialcytochrome-b gene of 12 taxa representingthree putative subspecies of Pseudobulweria,seven species in six othergenera of the Procellariidae(fulmars, petrels, and shearwaters),and onespecies each from the Hydrobatidae(storm-petrels) and Pelecanoidi- dae (diving-petrels).We alsoinclude published sequences for two otherpetrels (Procellaria cinereaand Macronectesgiganteus ) and use Diomedeaexulans and Pelecanuserythrorhynchos as outgroups.Based on thepronounced sequence divergence (5 to 5.5%)and separate phylo- genetichistory from othergenera that havebeen thought to be closelyrelated to or have beensynonymized with Pseudobulweria,we conclude that the genusis valid, and that the MascarenePetrel (Pseudobulweria aterrima) -

Appendix, French Names, Supplement
685 APPENDIX Part 1. Speciesreported from the A.O.U. Check-list area with insufficient evidencefor placementon the main list. Specieson this list havebeen reported (published) as occurring in the geographicarea coveredby this Check-list.However, their occurrenceis considered hypotheticalfor one of more of the following reasons: 1. Physicalevidence for their presence(e.g., specimen,photograph, video-tape, audio- recording)is lacking,of disputedorigin, or unknown.See the Prefacefor furtherdiscussion. 2. The naturaloccurrence (unrestrained by humans)of the speciesis disputed. 3. An introducedpopulation has failed to becomeestablished. 4. Inclusionin previouseditions of the Check-listwas basedexclusively on recordsfrom Greenland, which is now outside the A.O.U. Check-list area. Phoebastria irrorata (Salvin). Waved Albatross. Diornedeairrorata Salvin, 1883, Proc. Zool. Soc. London, p. 430. (Callao Bay, Peru.) This speciesbreeds on Hood Island in the Galapagosand on Isla de la Plata off Ecuador, and rangesat seaalong the coastsof Ecuadorand Peru. A specimenwas takenjust outside the North American area at Octavia Rocks, Colombia, near the Panama-Colombiaboundary (8 March 1941, R. C. Murphy). There are sight reportsfrom Panama,west of Pitias Bay, Dari6n, 26 February1941 (Ridgely 1976), and southwestof the Pearl Islands,27 September 1964. Also known as GalapagosAlbatross. ThalassarchechrysosWma (Forster). Gray-headed Albatross. Diornedeachrysostorna J. R. Forster,1785, M6m. Math. Phys. Acad. Sci. Paris 10: 571, pl. 14. (voisinagedu cerclepolaire antarctique & dansl'Ocean Pacifique= Isla de los Estados[= StatenIsland], off Tierra del Fuego.) This speciesbreeds on islandsoff CapeHorn, in the SouthAtlantic, in the southernIndian Ocean,and off New Zealand.Reports from Oregon(mouth of the ColumbiaRiver), California (coastnear Golden Gate), and Panama(Bay of Chiriqu0 are unsatisfactory(see A.O.U. -

Order PROCELLARIIFORMES: Albatrosses
Text extracted from Gill B.J.; Bell, B.D.; Chambers, G.K.; Medway, D.G.; Palma, R.L.; Scofield, R.P.; Tennyson, A.J.D.; Worthy, T.H. 2010. Checklist of the birds of New Zealand, Norfolk and Macquarie Islands, and the Ross Dependency, Antarctica. 4th edition. Wellington, Te Papa Press and Ornithological Society of New Zealand. Pages 64, 78-79 & 82-83. Order PROCELLARIIFORMES: Albatrosses, Petrels, Prions and Shearwaters Checklist Committee (1990) recognised three families within the Procellariiformes, however, four families are recognised here, with the reinstatement of Pelecanoididae, following many other recent authorities (e.g. Marchant & Higgins 1990, del Hoyo et al. 1992, Viot et al. 1993, Warham 1996: 484, Nunn & Stanley 1998, Dickinson 2003, Brooke 2004, Onley & Scofield 2007). The relationships of the families within the Procellariiformes are debated (e.g. Sibley & Alquist 1990, Christidis & Boles 1994, Nunn & Stanley 1998, Livezey & Zusi 2001, Kennedy & Page 2002, Rheindt & Austin 2005), so a traditional arrangement (Jouanin & Mougin 1979, Marchant & Higgins 1990, Warham 1990, del Hoyo et al. 1992, Warham 1996: 505, Dickinson 2003, Brooke 2004) has been adopted. The taxonomic recommendations (based on molecular analysis) on the Procellariiformes of Penhallurick & Wink (2004) have been heavily criticised (Rheindt & Austin 2005) and have seldom been followed here. Family PROCELLARIIDAE Leach: Fulmars, Petrels, Prions and Shearwaters Procellariidae Leach, 1820: Eleventh room. In Synopsis Contents British Museum 17th Edition, London: 68 – Type genus Procellaria Linnaeus, 1758. Subfamilies Procellariinae and Fulmarinae and shearwater subgenera Ardenna, Thyellodroma and Puffinus (as recognised by Checklist Committee 1990) are not accepted here given the lack of agreement about to which subgenera some species should be assigned (e.g. -

Petrelsrefs V1.1.Pdf
Introduction I have endeavoured to keep typos, errors, omissions etc in this list to a minimum, however when you find more I would be grateful if you could mail the details during 2017 & 2018 to: [email protected]. Please note that this and other Reference Lists I have compiled are not exhaustive and are best employed in conjunction with other sources. Grateful thanks to Killian Mullarney and Tom Shevlin (www.irishbirds.ie) for the cover images. All images © the photographers. Joe Hobbs Index The general order of species follows the International Ornithologists' Union World Bird List (Gill, F. & Donsker, D. (eds.) 2017. IOC World Bird List. Available from: http://www.worldbirdnames.org/ [version 7.3 accessed August 2017]). Version Version 1.1 (August 2017). Cover Main image: Bulwer’s Petrel. At sea off Madeira, North Atlantic. 14th May 2012. Picture by Killian Mullarney. Vignette: Northern Fulmar. Great Saltee Island, Co. Wexford, Ireland. 5th May 2008. Picture by Tom Shevlin. Species Page No. Antarctic Petrel [Thalassoica antarctica] 12 Beck's Petrel [Pseudobulweria becki] 18 Blue Petrel [Halobaena caerulea] 15 Bulwer's Petrel [Bulweria bulweri] 24 Cape Petrel [Daption capense] 13 Fiji Petrel [Pseudobulweria macgillivrayi] 19 Fulmar [Fulmarus glacialis] 8 Giant Petrels [Macronectes giganteus & halli] 4 Grey Petrel [Procellaria cinerea] 19 Jouanin's Petrel [Bulweria fallax] 27 Kerguelen Petrel [Aphrodroma brevirostris] 16 Mascarene Petrel [Pseudobulweria aterrima] 17 Parkinson’s Petrel [Procellaria parkinsoni] 23 Southern Fulmar [Fulmarus glacialoides] 11 Spectacled Petrel [Procellaria conspicillata] 22 Snow Petrel [Pagodroma nivea] 14 Tahiti Petrel [Pseudobulweria rostrata] 18 Westland Petrel [Procellaria westlandica] 23 White-chinned Petrel [Procellaria aequinoctialis] 20 1 Relevant Publications Beaman, M. -

AOS Classification Committee – North and Middle America Proposal Set 2018-B 17 January 2018
AOS Classification Committee – North and Middle America Proposal Set 2018-B 17 January 2018 No. Page Title 01 02 Split Fork-tailed Swift Apus pacificus into four species 02 05 Restore Canada Jay as the English name of Perisoreus canadensis 03 13 Recognize two genera in Stercorariidae 04 15 Split Red-eyed Vireo (Vireo olivaceus) into two species 05 19 Split Pseudobulweria from Pterodroma 06 25 Add Tadorna tadorna (Common Shelduck) to the Checklist 07 27 Add three species to the U.S. list 08 29 Change the English names of the two species of Gallinula that occur in our area 09 32 Change the English name of Leistes militaris to Red-breasted Meadowlark 10 35 Revise generic assignments of woodpeckers of the genus Picoides 11 39 Split the storm-petrels (Hydrobatidae) into two families 1 2018-B-1 N&MA Classification Committee p. 280 Split Fork-tailed Swift Apus pacificus into four species Effect on NACC: This proposal would change the species circumscription of Fork-tailed Swift Apus pacificus by splitting it into four species. The form that occurs in the NACC area is nominate pacificus, so the current species account would remain unchanged except for the distributional statement and notes. Background: The Fork-tailed Swift Apus pacificus was until recently (e.g., Chantler 1999, 2000) considered to consist of four subspecies: pacificus, kanoi, cooki, and leuconyx. Nominate pacificus is highly migratory, breeding from Siberia south to northern China and Japan, and wintering in Australia, Indonesia, and Malaysia. The other subspecies are either residents or short distance migrants: kanoi, which breeds from Taiwan west to SE Tibet and appears to winter as far south as southeast Asia. -

First Two Cases of Melanism in Cory's Shearwater Calonectris Diomedea
Bried et al.: Melanism in Cory’s Shearwater Calonectris diomedea 19 FIRST TWO CASES OF MELANISM IN CORY’S SHEARWATER CALONECTRIS DIOMEDEA JOËL BRIED1, HELDER FRAGA2, PASCUAL CALABUIG-MIRANDA3 & VERÓNICA C. NEVES4 1 Departamento de Oceanografia e Pescas, Centro do IMAR da Universidade dos Açores, 9901-862 Horta, Açores, Portugal ([email protected], [email protected]) 2 Secretaria Regional do Ambiente, Rua Cônsul Dabney, 9900-014 Horta, Açores, Portugal 3 Centro de Recuperación de Fauna Silvestre de Tafira, Cabildo de Gran Canaria, Spain 4 Ornithology Unit, IBLS, University of Glasgow, Graham Kerr Building, G12 8QQ, Glasgow, Scotland, UK Received 19 November 2004, accepted 8 April 2005 SUMMARY BRIED, J., FRAGA, H., CALABUIG-MIRANDA, P. & NEVES, V.C. 2005. First two cases of melanism in Cory’s Shearwater Calonectris diomedea. Marine Ornithology 33: 19-22. Although aberrant colourations occur in a great variety of animal species, their frequency of occurrence is low. Here, we report the first two observations of melanistic Cory’s Shearwaters Calonectris diomedea, in the Azores archipelago and on the Canary Islands. Because dark plumages may be associated with subordinate status within petrel flocks and with increased conspicuousness, melanistic individuals would have increased difficulties in obtaining food compared with their normally coloured conspecifics. This phenomenon might explain why melanism seems to occur less frequently than other aberrant colour patterns in this group. Key words: Melanism, Cory’s Shearwater, Calonectris diomedea, Procellariiformes INTRODUCTION individuals were found in 2003. The first individual was found near a pier at San Andrés on 30 October. The second observation Aberrant colourations have a low frequency of occurrence in occurred in a street of Horta on 21 November. -

A Bulwer's Petrel Bulweria Bulwerii in Victoria
AUSTRALIAN 114 BIRD WATCHER AUSTRALIAN BIRD WATCHER 1989, 13, 114-117 A Bulwer's Petrel Bulweria bulwerii in Victoria by MIKE CARTER1 and TIM REID2 130 Canadian Bay Road, Mt Eliza, Victoria 3930 2Flat 2/61 Fawkner Street, St Kilda, Victoria 3182 Summary A Victorian record of Bulwer's Petrel Bulweria bulwerii is documented and discussed in relation to previous reports in the Australian region and vagrancy in the Atlantic. The observation A Bulwer's Petrel Bulweria bulwerii was seen at sea near Portland in south-western Victoria on 14 September 1986. It was flying among massed feeding seabirds at 38 ~l'S, 141 "34 'E, five nautical miles south-south-east of Cape Nelson. The sounder aboard the 12.8 m long fishing vessel engaged on an RAOU Seabird Group excursion indicated the depth of the ocean floor to be 75 m. Thus we were above the continental shelf in inshore waters. The bird was under observation for only a few minutes just before 0830 h E.S.T., but was well seen and scrutinised for diagnostic characters when it was realised that it was either one of the all-dark 'sooty' storm-petrels Oceanodroma spp. or a Bulwer's Petrel. The closest it approached the boat was about 50 m. The light was dull because of the heavily overcast skies but as the bird passed to the south and west of our position, the sun was behind us so viewing conditions were advantageous. Although the bird hugged the surface of the sea, it was only occasionally lost from view behind waves until it changed direction, headed away from the boat and disappeared. -

Notes on Tubinares, Including Records Which Affect the A
58 MURrH¾,Notes on Tubinares. Jan.Auk NOTES ON TUBINARES, INCLUDING RECORDS WHICH AFFECT THE A. O. U. CHECK-LIST. BY ROBERT CUSHMAN MURPHY. 1. Thala.•.•arehe ehlororhyneho.• (Gmelin).YELLOW-NOSED •OLLYMAWK. The first North American record of this subantarctic albatross is basedupon a skinin the AmericanMuseum of Natural History (collectionof Dr. L. C. Sanford,No. 9835,). The specimenis an adult of unknown sex. It was collected near Seal Island, off MacbiasBay, Maine, on August1, 1913,by Mr. ErnestO. Joye, subsequentlycoming into the possessionof Mr. Allen L. Moses and, finally, that of Dr. Sanford. Seal Island is Canadianterri- tory, and sincethe localityin whichthe birdwas killed is on the internationalborder, south of Grand Manan, the recordconstitutes an addition to the local avifauna of both New Brunswick and Maine. The specimenclosely resembles others collected by the writer in the South Atlantic Ocean. The fact that its sex was not determined decreases the value of measurements, which are, however,as follows: Wing 482; tail 186; culmen 116; tarsus 78; middle toe with claw 114 min. Thalassarchechlororhynchos is of circumpolardistribution in the southern hemisphere. Its Atlantic breeding grounds are presumablyin the "Tristan da Cunha Subarea,"as recently definedin Dr. RobertoDabbene's admirable paper on the petrels and albatrosses of the South Atlantic. • Sufficient material is not available for a determinationof the subspeclficstatus of the Atlantic bird, or of its relationshipwith the "Thalassogeronexi- mius" of Verrill. 2. Calonectriskuhlii kuhlii (Bole). MEDITERRANEANSHEAR- WATER. While examiningseries of North Americanexamples of the large shearwaterdescribed by Cory as Puffinusborealis (Bull. Nuttall Orn. C1.Vol. 6, p: 84, 1881),the writerhas occasionally encountered • E1 Hornero, Vol. -

Black Petrels on Hauturu-Little Barrier Island 2015-16 Season
Black petrel (Procellaria parkinsoni) population study on Hauturu‐o‐Toi/Little Barrier Island, 2015/16 Black petrels (Procellaria parkinsoni) population study on Hauturu‐o‐Toi/Little Barrier Island, 2015/16. Elizabeth A. Bell1, Claudia P. Mischler2, Nikki MacArthur3 and Joanna L. Sim4 1. Corresponding author: Wildlife Management International Limited, PO Box 607, Blenheim 7240, New Zealand, Email: [email protected] 2. Wildlife Management International Limited, PO Box 607, Blenheim 7240, New Zealand 3. Wildlife Management International Limited, PO Box 607, Blenheim 7240, New Zealand 4. DabChickNZ, Levin, New Zealand This report was prepared by Wildlife Management International Limited for the Department of Conservation as fulfilment of the contract 4652‐2 (Black petrel population study at Little Barrier Island) dated 21 December 2015. 15 September 2016 Citation: This report should be cited as: Bell, E.A.; Mischler, C.P.; MacArthur, N.; Sim, J.L. 2016. Black petrel (Procellaria parkinsoni) population study on Hauturu‐o‐Toi/Little Barrier Island, 2015/16. Report to the Conservation Services Programme, Department of Conservation. Wellington, New Zealand. All photographs in this Report are copyright © WMIL unless otherwise credited, in which case the person or organization credited is the copyright holder. Frontispiece: Niki MacArthur (WMIL, banding a black petrel chick with Adam Clow and Ashe Pollock (Whitianga fishers) assisting, May 2016. Bell et al. 2016: Black petrels on Little Barrier Island (POP2015/01) ABSTRACT This report covers the population monitoring of black petrels, Procellaria parkinsoni, on Hauturu‐o‐Toi/Little Barrier Island in the 2015/16 breeding season. On Hauturu‐o‐Toi/Little Barrier Island, 149 study burrows were monitored, of which 92 were original study burrows established in 1997 by Mike Imber. -
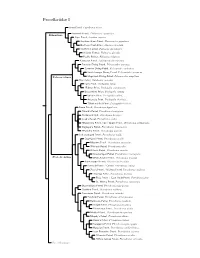
Procellariidae Species Tree
Procellariidae I Snow Petrel, Pagodroma nivea Antarctic Petrel, Thalassoica antarctica Fulmarinae Cape Petrel, Daption capense Southern Giant-Petrel, Macronectes giganteus Northern Giant-Petrel, Macronectes halli Southern Fulmar, Fulmarus glacialoides Atlantic Fulmar, Fulmarus glacialis Pacific Fulmar, Fulmarus rodgersii Kerguelen Petrel, Aphrodroma brevirostris Peruvian Diving-Petrel, Pelecanoides garnotii Common Diving-Petrel, Pelecanoides urinatrix South Georgia Diving-Petrel, Pelecanoides georgicus Pelecanoidinae Magellanic Diving-Petrel, Pelecanoides magellani Blue Petrel, Halobaena caerulea Fairy Prion, Pachyptila turtur ?Fulmar Prion, Pachyptila crassirostris Broad-billed Prion, Pachyptila vittata Salvin’s Prion, Pachyptila salvini Antarctic Prion, Pachyptila desolata ?Slender-billed Prion, Pachyptila belcheri Bonin Petrel, Pterodroma hypoleuca ?Gould’s Petrel, Pterodroma leucoptera ?Collared Petrel, Pterodroma brevipes Cook’s Petrel, Pterodroma cookii ?Masatierra Petrel / De Filippi’s Petrel, Pterodroma defilippiana Stejneger’s Petrel, Pterodroma longirostris ?Pycroft’s Petrel, Pterodroma pycrofti Soft-plumaged Petrel, Pterodroma mollis Gray-faced Petrel, Pterodroma gouldi Magenta Petrel, Pterodroma magentae ?Phoenix Petrel, Pterodroma alba Atlantic Petrel, Pterodroma incerta Great-winged Petrel, Pterodroma macroptera Pterodrominae White-headed Petrel, Pterodroma lessonii Black-capped Petrel, Pterodroma hasitata Bermuda Petrel / Cahow, Pterodroma cahow Zino’s Petrel / Madeira Petrel, Pterodroma madeira Desertas Petrel, Pterodroma -

Procellaria Parkinsoni) Using Morphometric Measurements and Discriminant Function Analysis
57 Notornis, 2015, Vol. 62: 57-62 0029-4470 © The Ornithological Society of New Zealand Inc. Sex determination of black petrels (Procellaria parkinsoni) using morphometric measurements and discriminant function analysis CLAUDIA P. MISCHLER* Wildlife Management International Limited, PO Box 607, Blenheim 7240, New Zealand ELIZABETH A. BELL Wildlife Management International Limited, PO Box 607, Blenheim 7240, New Zealand TODD J. LANDERS Auckland Council, Private Bag 92300, Auckland 1142, New Zealand TODD E. DENNIS School of Biological Sciences, University of Auckland, Auckland PB 92019, New Zealand Abstract Discriminant function analysis (DFA) is widely used to determine sex in the field from morphological measurements of bird species with monomorphic plumage. Sexual dimorphism was examined in black petrels (Procellaria parkinsoni) using 7 external measurements of adult birds breeding on Great Barrier Island, New Zealand. Males were significantly larger than females in absolute values of all measurements except for tarsus. Two stepwise DFA models were developed. The first used all 7 parameters, while the second model used only 6 parameters in order to increase sample size. Model one and two showed an 88 and 82% classification success, respectively, most likely due to the high overlap in measurements between males and females. These canonical functions were not accurate enough for field surveys, but may be improved using a larger and more representative sample size. Mischler, C.P.; Bell, E.A.; Landers, T.J.; Dennis, T.E. 2015. Sex determination of black petrels (Procellaria parkinsoni) using morphometric measurements and discriminant function analysis. Notornis 62 (2): 57-62. Keywords field measurements; sexing; conservation; population dynamics; black petrel INTRODUCTION et al.