Conditioning Trial Duration Affects Ethanol-Induced Conditioned Place Preference in Mice
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Gabab Regulation of Methamphetamine-Induced Associative Learning
Loyola University Chicago Loyola eCommons Dissertations Theses and Dissertations 2010 Gabab Regulation of Methamphetamine-Induced Associative Learning Robin Michelle Voigt Loyola University Chicago Follow this and additional works at: https://ecommons.luc.edu/luc_diss Part of the Pharmacology Commons Recommended Citation Voigt, Robin Michelle, "Gabab Regulation of Methamphetamine-Induced Associative Learning" (2010). Dissertations. 38. https://ecommons.luc.edu/luc_diss/38 This Dissertation is brought to you for free and open access by the Theses and Dissertations at Loyola eCommons. It has been accepted for inclusion in Dissertations by an authorized administrator of Loyola eCommons. For more information, please contact [email protected]. This work is licensed under a Creative Commons Attribution-Noncommercial-No Derivative Works 3.0 License. Copyright © 2010 Robin Michelle Voigt LOYOLA UNIVERSITY CHICAGO GABAB REGULATION OF METHAMPHETAMINE-INDUCED ASSOCIATIVE LEARNING A DISSERTATION SUBMITTED TO THE FACULTY OF THE GRADUATE SCHOOL IN CANDIDACY FOR THE DEGREE OF DOCTOR OF PHILOSOPHY PROGRAM IN MOLECULAR PHARMACOLOGY & THERAPEUTICS BY ROBIN MICHELLE VOIGT CHICAGO, IL DECEMBER 2010 Copyright by Robin Michelle Voigt, 2010 All rights reserved ACKNOWLEDGEMENTS Without the support of so many generous and wonderful individuals I would not have been able to be where I am today. First, I would like to thank my Mother for her belief that I could accomplish anything that I set my mind to. I would also like to thank my dissertation advisor, Dr. Celeste Napier, for encouraging and challenging me to be better than I thought possible. I extend gratitude to my committee members, Drs. Julie Kauer, Adriano Marchese, Micky Marinelli, and Karie Scrogin for their guidance and insightful input. -

Amphetamine Sensitization Alters Hippocampal Neuronal Morphology and Memory and Learning Behaviors
Molecular Psychiatry https://doi.org/10.1038/s41380-020-0809-2 ARTICLE Amphetamine sensitization alters hippocampal neuronal morphology and memory and learning behaviors 1,2,5 1,2 1 Luis Enrique Arroyo-García ● Hiram Tendilla-Beltrán ● Rubén Antonio Vázquez-Roque ● 1 3 3 3 1 Erick Ernesto Jurado-Tapia ● Alfonso Díaz ● Patricia Aguilar-Alonso ● Eduardo Brambila ● Eduardo Monjaraz ● 2 4 1 Fidel De La Cruz ● Antonio Rodríguez-Moreno ● Gonzalo Flores Received: 12 November 2019 / Revised: 29 May 2020 / Accepted: 3 June 2020 © The Author(s), under exclusive licence to Springer Nature Limited 2020 Abstract It is known that continuous abuse of amphetamine (AMPH) results in alterations in neuronal structure and cognitive behaviors related to the reward system. However, the impact of AMPH abuse on the hippocampus remains unknown. The aim of this study was to determine the damage caused by AMPH in the hippocampus in an addiction model. We reproduced the AMPH sensitization model proposed by Robinson et al. in 1997 and performed the novel object recognition test (NORt) to evaluate learning and memory behaviors. After the NORt, we performed Golgi–Cox staining, a 1234567890();,: 1234567890();,: stereological cell count, immunohistochemistry to determine the presence of GFAP, CASP3, and MT-III, and evaluated oxidative stress in the hippocampus. We found that AMPH treatment generates impairment in short- and long-term memories and a decrease in neuronal density in the CA1 region of the hippocampus. The morphological test showed an increase in the total dendritic length, but a decrease in the number of mature spines in the CA1 region. GFAP labeling increased in the CA1 region and MT-III increased in the CA1 and CA3 regions. -

Comparison Between Two Methodological Paradigms of Conditioned Place Preference with Methlyphenidate
East Tennessee State University Digital Commons @ East Tennessee State University Undergraduate Honors Theses Student Works 12-2013 Comparison between Two Methodological Paradigms of Conditioned Place Preference with Methlyphenidate. Bryce D. Watson East Tennessee State University Follow this and additional works at: https://dc.etsu.edu/honors Part of the Psychiatry and Psychology Commons Recommended Citation Watson, Bryce D., "Comparison between Two Methodological Paradigms of Conditioned Place Preference with Methlyphenidate." (2013). Undergraduate Honors Theses. Paper 89. https://dc.etsu.edu/honors/89 This Honors Thesis - Open Access is brought to you for free and open access by the Student Works at Digital Commons @ East Tennessee State University. It has been accepted for inclusion in Undergraduate Honors Theses by an authorized administrator of Digital Commons @ East Tennessee State University. For more information, please contact [email protected]. Watson 1 Comparison between Two Methodological Paradigms of Conditioned Place Preference with Methlyphenidate By Bryce Watson The Honors College Honors in Discipline Program East Tennessee State University Department of Psychology December 9, 2013 Russell Brown, Faculty Mentor David Harker, Faculty Reader Eric Sellers, Faculty Reader Watson 2 Abstract The aim of this thesis is to examine the mechanisms of Methylphenidate (MPH) on Conditioned Place Preference (CPP), a behavioral test of reward. The psychostimulant MPH is therapeutically used in the treatment of ADHD, but has been implicated in many pharmacological actions related to drug addiction and is considered to have abuse potential. Past work in our lab and others have shown substantial sex-differences in the neuropharmacological profile of MPH. Here a discussion of the relevant mechanisms of action of MPH and its relationship to neurotrophins and CPP are reviewed. -

D, Dopamine Receptor Activation Is Necessary for the Induction of Sensitization by Amphetamine in the Ventral Tegmental Area
The Journal of Neuroscience, April 1, 1996, 76(7):241 l-2420 D, Dopamine Receptor Activation Is Necessary for the Induction of Sensitization by Amphetamine in the Ventral Tegmental Area Paul Vezina Department of Psychiatry, The University of Chicago, Chicago, Illinois 6063 7 Repeated intermittent exposure to amphetamine produces injections of amphetamine. In the second experiment, locomo- long-term enhancements in the ability of this drug to produce tor sensitization induced by infusion of amphetamine into the locomotion and increase extracellular dopamine (DA) in the ventral tegmental area (WA) was blocked when these injections nucleus accumbens (NAcc). Three experiments were con- were preceded by systemic injections of SCH23390. Finally, in ducted to evaluate the role played by D, DA receptors in the experiment three, co-injecting SCH23390, but not its inactive production of these changes in response to amphetamine. Rats enantiomer, with amphetamine into the VTA during preexpo- were preexposed to amphetamine, alone or with a DA receptor sure prevented sensitization of the NAcc DA response to this antagonist, and tested for sensitization l-3 weeks after the last drug. These results indicate that while D, DA receptor activa- drug injection. On the test for sensitization, locomotor (experi- tion is not necessary for the induction of locomotor sensitiza- ments 1 and 2) and NAcc DA (experiment 3) responses of the tion to amphetamine, D, DA receptors located in the VTA play animals to a systemic amphetamine injection were assessed. In a critical role in the development of sensitized locomotor and the first experiment, systemic injections of the D, DA receptor NAcc DA response to this drug. -

Nonparaphilic Sexual Addiction Mark Kahabka
The Linacre Quarterly Volume 63 | Number 4 Article 2 11-1-1996 Nonparaphilic Sexual Addiction Mark Kahabka Follow this and additional works at: http://epublications.marquette.edu/lnq Part of the Ethics and Political Philosophy Commons, and the Medicine and Health Sciences Commons Recommended Citation Kahabka, Mark (1996) "Nonparaphilic Sexual Addiction," The Linacre Quarterly: Vol. 63: No. 4, Article 2. Available at: http://epublications.marquette.edu/lnq/vol63/iss4/2 Nonparaphilic Sexual Addiction by Mr. Mark Kahabka The author is a recent graduate from the Master's program in Pastoral Counseling at Saint Paul University in Ottawa, Ontario, Canada. Impulse control disorders of a sexual nature have probably plagued humankind from its beginnings. Sometimes classified today as "sexual addiction" or "nonparaphilic sexual addiction,"l it has been labeled by at least one professional working within the field as "'The World's Oldest/Newest Perplexity."'2 Newest, because for the most part, the only available data until recently has come from those working within the criminal justice system and as Patrick Carnes points out, "they never see the many addicts who have not been arrested."3 By definition, both paraphilic4 and nonparaphilic sexual disorders "involve intense sexual urges and fantasies" and which the "individual repeatedly acts on these urges or is highly distressed by them .. "5 Such disorders were at one time categorized under the classification of neurotic obsessions and compulsions, and thus were usually labeled as disorders of an obsessive compulsive nature. Since those falling into this latter category, however, perceive such obessions and compulsions as "an unwanted invasion of consciousness"6 (in contrast to sexual impulse control disorders, which are "inherently pleasurable and consciously desired"7) they are now placed under the "impulse control disorder" category.s To help clarify the distinction: The purpose of the compulsions is to reduce anxiety, which often stems from unwanted but intrusive thoughts. -

Molecular Mechanisms of Addiction
Molecular Mechanisms of Addiction Eric J. Nestler Nash Family Professor The Friedman Brain Institute Medical Model of Addiction • Pathophysiology - To identify changes that drugs produce in a vulnerable brain to cause addiction. • Individual Risk - To identify specific genes and non-genetic factors that determine an individual’s risk for (or resistance to) addiction. - About 50% of the risk for addiction is genetic. Only through an improved understanding of the biology of addiction will it be possible to develop better treatments and eventually cures and preventive measures. Scope of Drug Addiction • 25% of the U.S. population has a diagnosis of drug abuse or addiction. • 50% of U.S. high school graduates have tried an illegal drug; use of alcohol and tobacco is more common. • >$400 billion incurred annually in the U.S. by addiction: - Loss of life and productivity - Medical consequences (e.g., AIDS, lung cancer, cirrhosis) - Crime and law enforcement Diverse Chemical Substances Cause Addiction • Opiates (morphine, heroin, oxycontin, vicodin) • Cocaine • Amphetamine and like drugs (methamphetamine, methylphenidate) • MDMA (ecstasy) • PCP (phencyclidine or angel dust; also ketamine) • Marijuana (cannabinoids) • Tobacco (nicotine) • Alcohol (ethanol) • Sedative/hypnotics (barbiturates, benzodiazepines) Chemical Structures of Some Drugs of Abuse Cocaine Morphine Ethanol Nicotine ∆9-tetrahydrocannabinol Drugs of Abuse Use of % of US population as weekly users 100 25 50 75 0 Definition of Drug Addiction • Loss of control over drug use. • Compulsive drug seeking and drug taking despite horrendous adverse consequences. • Increased risk for relapse despite years of abstinence. Definition of Drug Addiction • Tolerance – reduced drug effect after repeated use. • Sensitization – increased drug effect after repeated use. -

The Addictive Potential of Sexual Behavior (Impulse) Review2
Page 1 of 9 Impulse: The Premier Journal for Undergraduate Publications in the Neurosciences Submitted for Publication January, 2018 The Addictive Potential of Sexual Behavior Heather Bool D’Youville College, Buffalo, New York This paper examines the addictive potential of sexual behavior through behavioral and neurophysiological mechanisms analogous to other formalized addictions. Sexual behavior refers to any action or thought preformed with the intention of sexual gratification, such as the consumption of explicit material, masturbation, fantasizing of sexual scenarios, and sexual intercourse. Addiction is defined by the presence of tolerance, preoccupation, withdrawal, dependence, and the continuation of behavior despite risk and/or harm. Sexual addiction demonstrates high relapse potential due to the frequency of reward-associated cues encountered in daily life, and the low effort and risk required for sexual pleasure. Currently, sexual addiction lacks a formal diagnosis despite behavioral, psychological, and physiological evidence. An official diagnosis recognized by a governing authority, such as the American Psychological Association, would offer greater access to treatment, funding for research, and exposure and education for the general public about this disorder. Abbreviations: None Keywords: Sexual Behavior; Addiction; Sexual Addiction; Neurophysiology; Behavioral Neuroscience Introduction “Sexual addiction” is an umbrella term Confusion remains regarding the for sexual impulsivity, sexual compulsivity, out- etiology and nosology of sexual addiction, of-control sexual behavior, hypersexual which has led to the lack of a universally behavior or disorder, sexually excessive accepted criterion and, more importantly, the behavior or disorder, Don Jaunism, satyriasis, absence of a formal diagnosis. A lack of and obsessive-compulsive sexual behavior operationalization of the disorder has severe (Beech et al., 2009; Karila et al., 2014; effects on research; due to the use of Rosenberg et al., 2014). -

The Role of Habituation in Social Fear by Suzanne N. Avery Dissertation
Slow to Warm Up: The Role of Habituation in Social Fear By Suzanne N. Avery Dissertation Submitted to the Faculty of the Graduate School of Vanderbilt University in partial fulfillment of the requirements for the degree of DOCTOR OF PHILOSOPHY in Neuroscience August, 2015 Nashville, Tennessee Approved: David Zald, Ph.D. Bunmi Olatunji, Ph.D. Brandon Ally, Ph.D. Jennifer Blackford, Ph.D. Copyright © 2015 by Suzanne N. Avery All Rights Reserved ii To my husband, Stacy, for his unending support, and my daughter, Sophie, for whom I do everything iii ACKNOWLEDGMENTS I would first and foremost like to thank my advisor, Dr. Jennifer Blackford, who is a truly exceptional scientist, teacher and mentor. She has encouraged me to pursue every opportunity for scientific achievement and has mentored me closely through each step. Her enthusiasm to help me become an excellent scientist and her generosity with her time and effort have made my graduate training an incredible experience. She has an incredible passion for science and possesses a brilliant combination of curiosity, creativity, motivation, intelligence and passion that inspires all of those who have the pleasure to work with her. I am eternally grateful for your mentorship—thank you is not enough. I would also like to thank the current and former members of the Blackford lab, including Jacqueline Clauss, Ross VanDerKlok, and Brittany Matthews, whose assistance and encouragement have made this work possible. My gratitude goes out to my dissertation committee, who have provided invaluable feedback and whose suggestions have significantly improved this project. Thank you for your insights and help Dr. -

Stress Sensitization Model
The Stress Sensitization Model Oxford Handbooks Online The Stress Sensitization Model Catherine B. Stroud The Oxford Handbook of Stress and Mental Health Edited by Kate Harkness and Elizabeth P. Hayden Subject: Psychology, Clinical Psychology Online Publication Date: Jun 2018 DOI: 10.1093/oxfordhb/9780190681777.013.16 Abstract and Keywords The stress sensitization model was developed to explain the mechanism through which the relationship between stress and affective disorder onsets changes across the course of the disorder. The model posits that individuals become sensitized to stress over time, such that the level of stress needed to trigger episode onsets becomes increasingly lower with successive episodes. The stress sensitization model has accrued empirical support in the context of major depression and to a lesser extent in bipolar spectrum disorders. Furthermore, expanding upon the original stress sensitization model, research also indicates that early adversity (i.e., early childhood experiences) sensitizes individuals to subsequent proximal stress, increasing risk for psychopathology. In this chapter, the theoretical background underlying the stress sensitization model is reviewed, and research evidence investigating stress sensitization is evaluated. In addition, moderators and mechanisms of stress sensitization effects are reviewed, and recommendations for future research are provided. Keywords: stress sensitization, kindling, early adversity, childhood maltreatment, life events The nature of the relationship between stress -

Disrupted Habituation in the Early Stage of Psychosis
Biological Psychiatry: CNNI Archival Report Disrupted Habituation in the Early Stage of Psychosis Suzanne N. Avery, Maureen McHugo, Kristan Armstrong, Jennifer U. Blackford, Neil D. Woodward, and Stephan Heckers ABSTRACT BACKGROUND: Learning and memory are impaired in schizophrenia. Some theories have proposed that one form of memory, habituation, is particularly impaired. Preliminary evidence suggests that memory impairment is associated with failed hippocampal habituation in patients with chronic schizophrenia. We studied how abnormal habituation of the hippocampus is related to relational memory deficits in the early stage of psychosis. METHODS: We measured hippocampal activity in 62 patients with early psychosis and 70 healthy individuals using functional magnetic resonance imaging. Habituation was defined as the slope of functional magnetic resonance imaging signal change to repeated presentations of faces and objects. Relational memory ability was measured as the slope of preferential viewing during a face-scene pair eye movement task outside the scanner. RESULTS: Patients with early psychosis showed impaired relational memory (p , .001) and less hippocampal habituation to objects (p = .01) than healthy control subjects. In the healthy control group, better relational memory was associated with faster anterior hippocampal habituation (faces, r = 2.28, p = .03). In contrast, patients with early psychosis showed no brain-behavior relationship (r = .12, p = .40). CONCLUSIONS: We found evidence for disrupted hippocampal habituation in the early stage of psychosis along with an altered association between hippocampal habituation and relational memory ability. These results suggest that neural habituation may provide a novel target for early cognitive interventions in psychosis. Keywords: First episode, Hippocampus, Novelty, Relational memory, Schizophrenia, Visual cortex https://doi.org/10.1016/j.bpsc.2019.06.007 Repetition is one of the most familiar memory tools. -
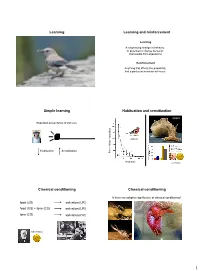
Learning Learning and Reinforcement Simple Learning Habituation and Sensitization Classical Conditioning Classical Conditioning
Learning Learning and reinforcement Learning A long-lasting change in behavior, or potential to change behavior, that results from experience Reinforcement Anything that affects the probability that a particular behavior will occur Simple learning Habituation and sensitization Aplysia Repeated presentation of stimulus chaffinch Habituation Sensitization Percent birds respondingbirds Percent Playbacks Eric Kandel Classical conditioning Classical conditioning Is there any adaptive significance of classical conditioning? food (US) salivation (UR) food (US) + tone (CS) salivation (UR) tone (CS) salivation (CR) Ivan Pavlov 1 Classical conditioning Classical conditioning ♂ ♀ blue gourami Karen Hollis Mount Holyoke Classical conditioning Classical conditioning How might classical conditioning work in nature? Courtship behavior by female (US) Courtship (UR) Courtship behavior by female (US) + sex-recognition cue (CS) Courtship (UR) Sex-recognition cue (CS) Courtship (CR) Japanese quail Heritability of conditioned response Gene × environment interactions Proboscis extension response conditioned to various odors Evidence of significant heritability Tryon Maze Dull and Bright rats Offspring score Offspring Parent score 2 Operant conditioning Laws of effect and exercise Thorndike Operant conditioning Operant conditioning Operant conditioning = association between Negative behavior and its consequences Positive reinforcement reinforcement Increase likelihood that behavior will occur B.F. Skinner Operant conditioning What factors affect conditioning? -

Habituation As a Neural Algorithm for Online Odor Discrimination
Habituation as a neural algorithm for online odor discrimination Yang Shena, Sanjoy Dasguptab, and Saket Navlakhaa,1 aSimons Center for Quantitative Biology, Cold Spring Harbor Laboratory, Cold Spring Harbor, NY 11724; and bComputer Science and Engineering Department, University of California San Diego, La Jolla, CA 92093 Edited by Timothy O’Leary, University of Cambridge, Cambridge, United Kingdom, and accepted by Editorial Board Member Gina G. Turrigiano April 13, 2020 (received for review September 5, 2019) Habituation is a form of simple memory that suppresses neural the extinction of the stimulus. This is in contrast with faster forms activity in response to repeated, neutral stimuli. This process is crit- of habituation, such as odorant receptor neuron (ORN) adapta- ical in helping organisms guide attention toward the most salient tion (17, 18) occurring on the order of hundreds of milliseconds, and novel features in the environment. Here, we follow known and longer forms of habituation, such as long-term habituation circuit mechanisms in the fruit fly olfactory system to derive a (16), which lasts for days. Different time scales of habituation simple algorithm for habituation. We show, both empirically and have also been described in the vertebrate olfactory system (1). analytically, that this algorithm is able to filter out redundant infor- Short-term habituation is of interest because it requires a rela- mation, enhance discrimination between odors that share a similar tively fast online mechanism for its initiation and decay, yet it is background, and improve detection of novel components in odor also stable enough to have a lasting effect. mixtures. Overall, we propose an algorithmic perspective on the Prior work has studied the mechanisms of short-term habitu- biological mechanism of habituation and use this perspective to ation in the mouse and honeybee olfactory circuits (19–22), but understand how sensory physiology can affect odor perception.