Habituation As a Neural Algorithm for Online Odor Discrimination
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Gabab Regulation of Methamphetamine-Induced Associative Learning
Loyola University Chicago Loyola eCommons Dissertations Theses and Dissertations 2010 Gabab Regulation of Methamphetamine-Induced Associative Learning Robin Michelle Voigt Loyola University Chicago Follow this and additional works at: https://ecommons.luc.edu/luc_diss Part of the Pharmacology Commons Recommended Citation Voigt, Robin Michelle, "Gabab Regulation of Methamphetamine-Induced Associative Learning" (2010). Dissertations. 38. https://ecommons.luc.edu/luc_diss/38 This Dissertation is brought to you for free and open access by the Theses and Dissertations at Loyola eCommons. It has been accepted for inclusion in Dissertations by an authorized administrator of Loyola eCommons. For more information, please contact [email protected]. This work is licensed under a Creative Commons Attribution-Noncommercial-No Derivative Works 3.0 License. Copyright © 2010 Robin Michelle Voigt LOYOLA UNIVERSITY CHICAGO GABAB REGULATION OF METHAMPHETAMINE-INDUCED ASSOCIATIVE LEARNING A DISSERTATION SUBMITTED TO THE FACULTY OF THE GRADUATE SCHOOL IN CANDIDACY FOR THE DEGREE OF DOCTOR OF PHILOSOPHY PROGRAM IN MOLECULAR PHARMACOLOGY & THERAPEUTICS BY ROBIN MICHELLE VOIGT CHICAGO, IL DECEMBER 2010 Copyright by Robin Michelle Voigt, 2010 All rights reserved ACKNOWLEDGEMENTS Without the support of so many generous and wonderful individuals I would not have been able to be where I am today. First, I would like to thank my Mother for her belief that I could accomplish anything that I set my mind to. I would also like to thank my dissertation advisor, Dr. Celeste Napier, for encouraging and challenging me to be better than I thought possible. I extend gratitude to my committee members, Drs. Julie Kauer, Adriano Marchese, Micky Marinelli, and Karie Scrogin for their guidance and insightful input. -

The Role of Habituation in Social Fear by Suzanne N. Avery Dissertation
Slow to Warm Up: The Role of Habituation in Social Fear By Suzanne N. Avery Dissertation Submitted to the Faculty of the Graduate School of Vanderbilt University in partial fulfillment of the requirements for the degree of DOCTOR OF PHILOSOPHY in Neuroscience August, 2015 Nashville, Tennessee Approved: David Zald, Ph.D. Bunmi Olatunji, Ph.D. Brandon Ally, Ph.D. Jennifer Blackford, Ph.D. Copyright © 2015 by Suzanne N. Avery All Rights Reserved ii To my husband, Stacy, for his unending support, and my daughter, Sophie, for whom I do everything iii ACKNOWLEDGMENTS I would first and foremost like to thank my advisor, Dr. Jennifer Blackford, who is a truly exceptional scientist, teacher and mentor. She has encouraged me to pursue every opportunity for scientific achievement and has mentored me closely through each step. Her enthusiasm to help me become an excellent scientist and her generosity with her time and effort have made my graduate training an incredible experience. She has an incredible passion for science and possesses a brilliant combination of curiosity, creativity, motivation, intelligence and passion that inspires all of those who have the pleasure to work with her. I am eternally grateful for your mentorship—thank you is not enough. I would also like to thank the current and former members of the Blackford lab, including Jacqueline Clauss, Ross VanDerKlok, and Brittany Matthews, whose assistance and encouragement have made this work possible. My gratitude goes out to my dissertation committee, who have provided invaluable feedback and whose suggestions have significantly improved this project. Thank you for your insights and help Dr. -

Disrupted Habituation in the Early Stage of Psychosis
Biological Psychiatry: CNNI Archival Report Disrupted Habituation in the Early Stage of Psychosis Suzanne N. Avery, Maureen McHugo, Kristan Armstrong, Jennifer U. Blackford, Neil D. Woodward, and Stephan Heckers ABSTRACT BACKGROUND: Learning and memory are impaired in schizophrenia. Some theories have proposed that one form of memory, habituation, is particularly impaired. Preliminary evidence suggests that memory impairment is associated with failed hippocampal habituation in patients with chronic schizophrenia. We studied how abnormal habituation of the hippocampus is related to relational memory deficits in the early stage of psychosis. METHODS: We measured hippocampal activity in 62 patients with early psychosis and 70 healthy individuals using functional magnetic resonance imaging. Habituation was defined as the slope of functional magnetic resonance imaging signal change to repeated presentations of faces and objects. Relational memory ability was measured as the slope of preferential viewing during a face-scene pair eye movement task outside the scanner. RESULTS: Patients with early psychosis showed impaired relational memory (p , .001) and less hippocampal habituation to objects (p = .01) than healthy control subjects. In the healthy control group, better relational memory was associated with faster anterior hippocampal habituation (faces, r = 2.28, p = .03). In contrast, patients with early psychosis showed no brain-behavior relationship (r = .12, p = .40). CONCLUSIONS: We found evidence for disrupted hippocampal habituation in the early stage of psychosis along with an altered association between hippocampal habituation and relational memory ability. These results suggest that neural habituation may provide a novel target for early cognitive interventions in psychosis. Keywords: First episode, Hippocampus, Novelty, Relational memory, Schizophrenia, Visual cortex https://doi.org/10.1016/j.bpsc.2019.06.007 Repetition is one of the most familiar memory tools. -
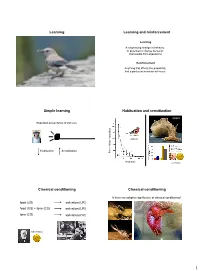
Learning Learning and Reinforcement Simple Learning Habituation and Sensitization Classical Conditioning Classical Conditioning
Learning Learning and reinforcement Learning A long-lasting change in behavior, or potential to change behavior, that results from experience Reinforcement Anything that affects the probability that a particular behavior will occur Simple learning Habituation and sensitization Aplysia Repeated presentation of stimulus chaffinch Habituation Sensitization Percent birds respondingbirds Percent Playbacks Eric Kandel Classical conditioning Classical conditioning Is there any adaptive significance of classical conditioning? food (US) salivation (UR) food (US) + tone (CS) salivation (UR) tone (CS) salivation (CR) Ivan Pavlov 1 Classical conditioning Classical conditioning ♂ ♀ blue gourami Karen Hollis Mount Holyoke Classical conditioning Classical conditioning How might classical conditioning work in nature? Courtship behavior by female (US) Courtship (UR) Courtship behavior by female (US) + sex-recognition cue (CS) Courtship (UR) Sex-recognition cue (CS) Courtship (CR) Japanese quail Heritability of conditioned response Gene × environment interactions Proboscis extension response conditioned to various odors Evidence of significant heritability Tryon Maze Dull and Bright rats Offspring score Offspring Parent score 2 Operant conditioning Laws of effect and exercise Thorndike Operant conditioning Operant conditioning Operant conditioning = association between Negative behavior and its consequences Positive reinforcement reinforcement Increase likelihood that behavior will occur B.F. Skinner Operant conditioning What factors affect conditioning? -

Posttraumatic Stress Disorder DSM IV to 5 Subcategories (4 to 5)
Emory Medicine: Strategic ImplementingRepositioning an Early 2013- 2017Intervention for the Prevention of PTSD in Emergency Department Patients Barbara Olasov Rothbaum, Ph.D., ABPP Paul A. Janssen Chair in Neuropsychopharmacology Director, Trauma and Anxiety Recovery Program Associate Vice Chair of Clinical Research Professor, Department of Psychiatry Emory University School of Medicine Disclosure This study was supported by National Institute of Mental Health Grant Numbers R34 MH083078 and R01 MH071537, and the Emory Center for Injury Control, Center for Disease Control Grant Number 5R49CE001494 and a NARSAD Distinguished Investigator award to Dr. Rothbaum. The authors report no biomedical financial interests or potential conflicts of interest. Recognition and Description of Posttraumatic Stress Disorder DSM IV to 5 subcategories (4 to 5) Criteria DSM-IV DSM-5 A Trauma Trauma B reexperiencing intrusion C Avoidance and avoidance numbing D hyperarousal Negative alterations in cognitions and mood E - Marked alterations in arousal and reactivity A Prospective Examination of PTSD in Rape Victims Percent of Victims with PTS 100 94 80 79 70 64 59 55 60 53 53 45 48 47 41 40 Percent Percent (%) 20 0 1 2 3 4 5 6 7 8 9 10 11 12 Assessment Rothbaum BO et al. J Traumatic Stress. 1992;5(3):463; NIMH Grant No. R01MH42178 Severity of PTSD Symptoms Non-PTS PTS 30 25 20 15 10 5 0 1 2 3 4 5 6 7 8 9 10 11 12 Assessment Rothbaum BO et al. J Traumatic Stress. 1992;5(3):464; NIMH Grant No. R01MH42178 Extinction and Habituation • We view PTSD as a disorder of extinction McSweeney FK, Swindell S. -

The Effect of Habituation and Changes in Cognition on Anxious Children's
Louisiana State University LSU Digital Commons LSU Master's Theses Graduate School 2014 The effect of habituation and changes in cognition on anxious children's performance on the WISC- IV Franziska Noack Louisiana State University and Agricultural and Mechanical College Follow this and additional works at: https://digitalcommons.lsu.edu/gradschool_theses Part of the Psychology Commons Recommended Citation Noack, Franziska, "The effect of habituation and changes in cognition on anxious children's performance on the WISC-IV" (2014). LSU Master's Theses. 988. https://digitalcommons.lsu.edu/gradschool_theses/988 This Thesis is brought to you for free and open access by the Graduate School at LSU Digital Commons. It has been accepted for inclusion in LSU Master's Theses by an authorized graduate school editor of LSU Digital Commons. For more information, please contact [email protected]. THE EFFECT OF HABITUATION AND CHANGES IN COGNITION ON ANXIOUS CHILDREN’S PERFORMANCE ON THE WISC-IV A Thesis Submitted to the Graduate Faculty of the Louisiana State University and Agricultural and Mechanical College in partial fulfillment of the requirements for the degree of Master of Arts in The Department of Psychology by Franziska Noack B.S., University of Houston-Clear Lake, 2012 May 2014 To my husband Daniel NoackLeSage and my parents Ilona and Ralf Noack. Thank you for your continuous support, kind words of encouragement, and loving patience. ------ An meinen Mann Daniel NoackLeSage und meine Eltern, Ilona und Ralf Noack. Danke für eure grenzenlose Unterstützung, eure freundlichen Worte der Ermutigung, und euer liebevolles Verständnis. ii ACKNOWLEDGEMENTS I would like to express my appreciation for the support and time allocated to this project by my advisor and mentor Thompson E. -

Conditioning Trial Duration Affects Ethanol-Induced Conditioned Place Preference in Mice
Animal Leaming & Behavior 1992, 20 (2), 187-194 Conditioning trial duration affects ethanol-induced conditioned place preference in mice CHRISTOPHER L. CUNNINGHAM and LIESL K. PRATHER Oregon Health Seiences University, Portland, Oregon The present experiments were designed to determine the efIect of conditioning trial duration on strength of ethanol-induced conditioned place preference in mice. In a counterbalanced, dif ferential conditioning procedure, DBA/2J mice received four pairings of a distinctive tactile (floor) stimulus with injection of ethanol (2 g/kg); a different floor stimulus was paired with saline. Dif ferent groups were exposed to the floor stimuli for 5, 15, or 30 min after injection. Conditioned place preference was inversely related to trial duration, with mice in the 5-, 15-,and 30-min groups, spending 83%,74%, and 66% oftheir time, respectively, on the ethanol-paired floor during a choice test. This outcome was replicated in a second experiment, which also showed that context famil iarity can influence conditioned place preference. In general, these findings suggest that ethanol's rewarding efIect is greatest shortly after injection. Study of the rewarding pbarmacological effect of 1989; van der Kooy, O'Shaughnessy, Mucba, & Kalant, ethanol has been limited primarily to examination of 1983). In those few instances where ethanol has produced ethanol drinking and preference (Myers & Veale, 1972) conditioned place preference, magnitude of preference is or operant self-administration by various routes (Meisch, rather -

Fear Conditioning in Posttraumatic Stress Disorder: Evidence for Delayed Extinction of Autonomic, Experiential, and Behavioural Responses
ARTICLE IN PRESS Behaviour Research and Therapy 45 (2007) 2019–2033 www.elsevier.com/locate/brat Fear conditioning in posttraumatic stress disorder: Evidence for delayed extinction of autonomic, experiential, and behavioural responses Jens BlechertÃ, Tanja Michael, Noortje Vriends, Ju¨rgen Margraf, Frank H. Wilhelm Department of Clinical Psychology and Psychotherapy, Institute for Psychology, University of Basel, Missionsstrasse 60/62, CH-4055 Basel, Switzerland Received 29 August 2006; received in revised form 26 February 2007; accepted 28 February 2007 Abstract Aversive conditioning has been proposed as an important factor involved in the etiology of posttraumatic stress disorder (PTSD). However, it is not yet fully understood exactly which learning mechanisms are characteristic for PTSD. PTSD patients (n ¼ 36), and healthy individuals with and without trauma exposure (TE group, n ¼ 21; nTE group, n ¼ 34), underwent a differential fear conditioning experiment consisting of habituation, acquisition, and extinction phases. An electrical stimulus served as the unconditioned stimulus (US), and two neutral pictures as conditioned stimuli (CSþ, paired; CSÀ, unpaired). Conditioned responses were quantified by skin conductance responses (SCRs), subjective ratings of CS valence and US-expectancy, and a behavioural test. In contrast to the nTE group, PTSD patients showed delayed extinction of SCRs to the CSþ. Online ratings of valence and US-expectancy as well as the behavioural test confirmed this pattern. These findings point to a deficit in extinction learning and highlight the role of affective valence appraisals and cognitive biases in PTSD. In addition, there was some evidence that a subgroup of PTSD patients had difficulties in learning the CS–US contingency, thereby providing preliminary evidence of reduced discrimination learning. -

Zebrafish and Conditioned Place Preference: a Translational Model of Drug Reward
The University of Southern Mississippi The Aquila Digital Community Master's Theses Summer 8-1-2015 Zebrafish and Conditioned Place Preference: A Translational Model of Drug Reward Adam Douglas Collier University of Southern Mississippi Follow this and additional works at: https://aquila.usm.edu/masters_theses Part of the Biological Psychology Commons Recommended Citation Collier, Adam Douglas, "Zebrafish and Conditioned Place Preference: A Translational Model of Drug Reward" (2015). Master's Theses. 132. https://aquila.usm.edu/masters_theses/132 This Masters Thesis is brought to you for free and open access by The Aquila Digital Community. It has been accepted for inclusion in Master's Theses by an authorized administrator of The Aquila Digital Community. For more information, please contact [email protected]. ZEBRAFISH AND CONDITIONED PLACE PREFERENCE: A TRANSLATIONAL MODEL OF DRUG REWARD by Adam Douglas Collier A Thesis Submitted to the Graduate School of The University of Southern Mississippi in Partial Fulfillment of the Requirements for the Degree of Master of Arts Approved: _____________________________________ Dr. David Echevarria, Committee Chair Associate Professor, Psychology _____________________________________ Dr. Richard Mohn, Committee Member Associate Professor, Educational Studies and Research _____________________________________ Dr. Brad Dufrene, Committee Member Associate Professor, Psychology _____________________________________ Dr. Peter McLaughlin, Committee Member Professor, Psychology, Edinboro University of Pennsylvania _____________________________________ Dr. Karen S. Coats Dean of the Graduate School August 2015 ABSTRACT ZEBRAFISH AND CONDITIONED PLACE PREFERENCE: A TRANSLATIONAL MODEL OF DRUG REWARD by Adam Douglas Collier August 2015 Addiction and substance abuse commonly lead to negative outcomes such as damaged health, domestic violence, child abuse, failure in school, and loss of employment. -

When Anxiety Affects Learning: How to Help Children with School-Related Anxiety
When Anxiety Affects Learning: How to Help Children with School-Related Anxiety March 27, 2017 Jonathan Dalton, Ph.D. Center for Anxiety and Behavioral Change Why this is so important Children and Adolescents . Median age of onset 11 – earliest of all forms of psychopathology . 8 % of children between ages 13 and 18 currently have an anxiety disorder . 31.9% will have an anxiety disorder between the ages of 13 and 18 . 8.3% will have “severe” anxiety disorder . Only 18 % of these teens receive treatment Adolescents Girls . 38% of girls will have at least one anxiety disorder between the ages of 13 and 18, (compared with 26.1% for boys) . Compared with 4.2% of girls will have ADHD, 10.2% with have a substance abuse disorder, 3.8% will have an eating disorder Comorbidity of Anxiety and Learning Differences . For children with a Specific Learning Disability, 28.8% meet criteria for an Anxiety Disorder . For children with a Nonspecific Learning Disability, 16.4% meet criteria for an Anxiety Disorder . For children with ADHD, 38.7% have comorbid anxiety disorder Adults . 28.8 percent lifetime prevalence . Most common category of mental health disorders . 18 % of adults currently have an anxiety disorder (40 million) . $42 billion in annual health costs Adaptive Anxiety vs. Disordered Anxiety Adaptive Anxiety Disordered Anxiety . Keeps us safe . Results in functional impairment . A response to real danger . Equivalent to a “false alarm” . Prevents the repeating of . Leads to unnecessary mistakes avoidance “If it made sense, it wouldn’t be a disorder” . 3797 ways to have a panic attack (4 of 12 symptoms are required) . -

Habituation of Distress and Craving During Treatment As Predictors of Change in PTSD Symptoms and Substance Use Severity Christal L
View metadata, citation and similar papers at core.ac.uk brought to you by CORE provided by University of Kentucky University of Kentucky UKnowledge Psychology Faculty Publications Psychology 3-2017 Habituation of Distress and Craving During Treatment as Predictors of Change in PTSD Symptoms and Substance Use Severity Christal L. Badour University of Kentucky, [email protected] Julianne C. Flanagan Medical University of South Carolina Daniel F. Gros Medical University of South Carolina Therese Killeen Medical University of South Carolina Irene Pericot-Valverde University of Oviedo, Spain See next page for additional authors Right click to open a feedback form in a new tab to let us know how this document benefits oy u. Follow this and additional works at: https://uknowledge.uky.edu/psychology_facpub Part of the Psychology Commons, and the Substance Abuse and Addiction Commons Repository Citation Badour, Christal L.; Flanagan, Julianne C.; Gros, Daniel F.; Killeen, Therese; Pericot-Valverde, Irene; Korte, Kristina J.; Allan, Nicholas P.; and Back, Sudie E., "Habituation of Distress and Craving During Treatment as Predictors of Change in PTSD Symptoms and Substance Use Severity" (2017). Psychology Faculty Publications. 159. https://uknowledge.uky.edu/psychology_facpub/159 This Article is brought to you for free and open access by the Psychology at UKnowledge. It has been accepted for inclusion in Psychology Faculty Publications by an authorized administrator of UKnowledge. For more information, please contact [email protected]. Authors Christal L. Badour, Julianne C. Flanagan, Daniel F. Gros, Therese Killeen, Irene Pericot-Valverde, Kristina J. Korte, Nicholas P. Allan, and Sudie E. Back Habituation of Distress and Craving During Treatment as Predictors of Change in PTSD Symptoms and Substance Use Severity Notes/Citation Information Published in Journal of Consulting and Clinical Psychology, v. -

CBT for OCD: Habituation Or Cognitive Shift?
The Cognitive Behaviour Therapist (2014), vol. 7, e6, page 1 of 16 PRACTICE ARTICLE doi:10.1017/S1754470X14000063 CBT for OCD: habituation or cognitive shift? Lottie Morris1∗ and Jim Nightingale2 1Department of Psychology, 6W 0.9, University of Bath, Claverton Down, Bath, UK 2Psychological Therapies Service, Cedar House, Blackberry Hill Hospital, Manor Road, Fishponds, Bristol, UK Received 30 July 2013; Accepted 11 April 2014 Abstract. Cognitive Behavioural Therapy (CBT) including Exposure and Response Prevention (ERP) is recommended by NICE as the psychological treatment of choice for obsessive compulsive disorder (OCD). Twenty-five percent of OCD patients refuse ERP, and many psychologists advocate formulation-driven cognitive therapy, including ERP, as opposed to ERP alone. However, a recent meta-analysis suggested there is insufficient evidence to suggest ERP is improved by cognitive methods. This paper proposes to contribute to this debate by providing a detailed description of the treatment of a patient with intrusive cognitions of a sexual nature, who was treated successfully using behavioural experiments designed to test cognitions, rather than ERP. This is, arguably, the way in which most cognitive behavioural therapists would work with someone with OCD. However, this approach is not reflected in the literature at present. The authors report the patient’s feedback that therapeutic change was brought about through cognitive shift, as a result of the formulation-driven behavioural experiments. Key words: Behavioural cognitive therapy, belief modification, CBT, cognition, OCD. Theoretical and research basis for therapy This paper begins by reviewing pertinent literature relating to this case. Anna’s presenting problem is then described, along with the cognitive behavioural hypotheses developed during treatment.