Dishabituation and Sensitization Emerge As Separate Processes During Development in Ap/Ysia
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

A Psychological Approach to Musical Form: the Habituation–Fluency Theory of Repetition
A Psychological Approach to Musical Form: The Habituation–Fluency Theory of Repetition David Huron With the possible exception of dance and meditation, there appears to be nothing else in common human experience that is comparable to music in its repetitiveness (Kivy 1993; Ockelford 2005; Margulis 2013). Narrative arti- facts like movies, novels, cartoon strips, stories, and speeches have much less internal repetition. Even poetry is less repetitive than music. Occasionally, architecture can approach music in repeating some elements, but only some- times. There appears to be no visual analog to the sort of trance–inducing music that can engage listeners for hours. Although dance and meditation may be more repetitive than music, dance is rarely performed in the absence of music, and meditation tellingly relies on imagining a repeated sound or mantra (Huron 2006: 267). Repetition can be observed in music from all over the world (Nettl 2005). In much music, a simple “strophic” pattern is evident in which a single phrase or passage is repeated over and over. When sung, it is common for successive repetitions to employ different words, as in the case of strophic verses. However, it is also common to hear the same words used with each repetition. In the Western art–music tradition, internal patterns of repetition are commonly discussed under the rubric of form. Writing in The Oxford Companion to Music, Percy Scholes characterized musical form as “a series of strategies designed to find a successful mean between the opposite extremes of unrelieved repetition and unrelieved alteration” (1977: 289). Scholes’s characterization notwithstanding, musical form entails much more than simply the pattern of repetition. -
Modeling the Dishabituation Hierarchy: the Role of the Primordial Hippocampus*
Biol. Cybern. 67, 535-544 (1992) Biological Cybemetics Springer-Verlag 1992 Modeling the dishabituation hierarchy: The role of the primordial hippocampus* DeLiang Wang and Michael A. Arbib Center for Neural Engineering, University of Southern California, Los Angeles, CA 90089-2520, USA Received January 30, 1992/accepted in revised form April 6, 1992 Abstract. We present a neural model for the organiza- orienting response to a certain stimulus applied at a tion and neural dynamics of the medial pallium, the given location, the response can be released by the same toad's homolog of mammalian hippocampus. A neural stimulus applied at a different retinal locus (Eikmanns mechanism, called cumulative shrinking, is proposed 1955; Ewert and Ingle 1971). (2) Hierarchical stimulus for mapping temporal responses from the anterior tha- specificity. Another stimulus given at the same locus lamus into a form of population coding referenced by may restore the response habituated by a previous spatial positions. Synaptic plasticity is modeled as an stimulus. Only certain stimuli can dishabituate a previ- interaction of two dynamic processes which simulates ously habituated response. Ewert and Kehl (1978) acquisition and both short-term and long-term forget- demonstrated that this dishabituation forms a hierarchy ting. The structure of the medial pallium model plus the (Fig. 1A), where a stimulus can dishabituate the habitu- plasticity model allows us to provide an account of the ated responses of another stimulus if the latter is lower neural mechanisms of habituation and dishabituation. in the hierarchy. Computer simulations demonstrate a remarkable match A model developed by Wang and Arbib (1991a) between the model performance and the original exper- showed how a group of cells in the anterior thalamus imental data on which the dishabituation hierarchy was (AT) could respond to stimuli higher in the hierarchy based. -

Gabab Regulation of Methamphetamine-Induced Associative Learning
Loyola University Chicago Loyola eCommons Dissertations Theses and Dissertations 2010 Gabab Regulation of Methamphetamine-Induced Associative Learning Robin Michelle Voigt Loyola University Chicago Follow this and additional works at: https://ecommons.luc.edu/luc_diss Part of the Pharmacology Commons Recommended Citation Voigt, Robin Michelle, "Gabab Regulation of Methamphetamine-Induced Associative Learning" (2010). Dissertations. 38. https://ecommons.luc.edu/luc_diss/38 This Dissertation is brought to you for free and open access by the Theses and Dissertations at Loyola eCommons. It has been accepted for inclusion in Dissertations by an authorized administrator of Loyola eCommons. For more information, please contact [email protected]. This work is licensed under a Creative Commons Attribution-Noncommercial-No Derivative Works 3.0 License. Copyright © 2010 Robin Michelle Voigt LOYOLA UNIVERSITY CHICAGO GABAB REGULATION OF METHAMPHETAMINE-INDUCED ASSOCIATIVE LEARNING A DISSERTATION SUBMITTED TO THE FACULTY OF THE GRADUATE SCHOOL IN CANDIDACY FOR THE DEGREE OF DOCTOR OF PHILOSOPHY PROGRAM IN MOLECULAR PHARMACOLOGY & THERAPEUTICS BY ROBIN MICHELLE VOIGT CHICAGO, IL DECEMBER 2010 Copyright by Robin Michelle Voigt, 2010 All rights reserved ACKNOWLEDGEMENTS Without the support of so many generous and wonderful individuals I would not have been able to be where I am today. First, I would like to thank my Mother for her belief that I could accomplish anything that I set my mind to. I would also like to thank my dissertation advisor, Dr. Celeste Napier, for encouraging and challenging me to be better than I thought possible. I extend gratitude to my committee members, Drs. Julie Kauer, Adriano Marchese, Micky Marinelli, and Karie Scrogin for their guidance and insightful input. -

Journal of Neurobiology, Parametric Studies of The
JOURNAL OF NEUROBIOLOGY,VOL. 1, NO. 3, PP. 345-360 PARAMETRIC STUDIES OF THE RESPONSE DECREMENT PRODUCED BY MECHANICAL STIMULI IN THE PROTOZOAN, Stentor coeruleus DAVID C. WOOD* Brain Research Laboratory, The University of Michigan, Ann Arbor, Michigan 43104 SUMMARY A decrement in the probability of eliciting a response is observed dur- ing the course of repetitive stimulation in many response systems of both metazoa and protozoa. Those forms of metazoan response decre- ment called habituation have recently been characterized behaviorally. In the studies reported here, the response decrement of the contractile protozoan, Stentor coeruleus, to repeated mechanical stimulation was systemically characterized to provide a comparison with metazoan habit- uation. This change in response probability was closely approximated by a negative exponential function after the first few stimuli. Animals responded to parametric variations of stimulus amplitude, interstimulus interval and retention period in a manner paralleling that observed in metazoa. However, neither interpolated large amplitude mechanical stimuli nor suprathreshold electrical stimuli produced dishabituation in Stentor though these stimuli are among the most effective dishabituating stimuli for metazoan tactile response systems. Behavioral analysis of the response decrement proceeded on the assumption that Stentor con- tains receptor and effector mechanisms only. Since the response decre- ment was not correlated with a change in responsiveness to electrical stimuli, the ability of the animal to contract appears to be unaltered by the process producing the response decrement. On the other hand, weak mechanical prestimuli were found to increase the rate of the response decrement which suggests that a process of receptor adaption is opera- tive. -

Orienting Response Reinstatement and Dishabituation: the Effects of Substituting
Orienting Response Reinstatement and Dishabituation: The Effects of Substituting, Adding and Deleting Components of Nonsignificant Stimuli Gershon Ben-Shakar, Itamar Gati, Naomi Ben-Bassat, and Galit Sniper The Hebrew University of Jerusalem Acknowledgments: This research was supported by The Israel Science Foundation founded by The Academy for Sciences and Humanities. We thank Dana Ballas, Rotem Shelef, Limor Bar, and Erga Sinai for their help in the data collection. We also thank John Furedy and an anonymous reviewer for the helpful comments on an earlier version of this manuscript. The article was written while the first author was on a sabbatical leave at Brandeis University. We wish to thank the Psychology Department at Brandeis University for the facilities and the help provided during this period. Address requests for reprints to: Prof. Gershon Ben-Shakhar Department of Psychology, The Hebrew University, Jerusalem, 91905, ISRAEL Running Title: OR Reinstatement and Dishabituation 2 ABSTRACT This study examined the prediction that stimulus novelty is negatively related to the measure of common features, shared by the stimulus input and representations of preceding events, and positively related to the measure of their distinctive features. This prediction was tested in two experiments, which used sequences of nonsignificant verbal and pictorial compound stimuli. A test stimulus (TS) was presented after 9 repetitions of a standard stimulus (SS), followed by 2 additional repetitions of SS. TS was created by either substituting 0, 1, or 2 stimulus components of SS (Experiment 1), or by either adding or deleting 0, 1, or 2 components of SS (Experiment 2). The dependent measure was the electrodermal component of the OR to both TS (OR reinstatement) and SS that immediately followed TS (dishabituation). -

The Role of Habituation in Social Fear by Suzanne N. Avery Dissertation
Slow to Warm Up: The Role of Habituation in Social Fear By Suzanne N. Avery Dissertation Submitted to the Faculty of the Graduate School of Vanderbilt University in partial fulfillment of the requirements for the degree of DOCTOR OF PHILOSOPHY in Neuroscience August, 2015 Nashville, Tennessee Approved: David Zald, Ph.D. Bunmi Olatunji, Ph.D. Brandon Ally, Ph.D. Jennifer Blackford, Ph.D. Copyright © 2015 by Suzanne N. Avery All Rights Reserved ii To my husband, Stacy, for his unending support, and my daughter, Sophie, for whom I do everything iii ACKNOWLEDGMENTS I would first and foremost like to thank my advisor, Dr. Jennifer Blackford, who is a truly exceptional scientist, teacher and mentor. She has encouraged me to pursue every opportunity for scientific achievement and has mentored me closely through each step. Her enthusiasm to help me become an excellent scientist and her generosity with her time and effort have made my graduate training an incredible experience. She has an incredible passion for science and possesses a brilliant combination of curiosity, creativity, motivation, intelligence and passion that inspires all of those who have the pleasure to work with her. I am eternally grateful for your mentorship—thank you is not enough. I would also like to thank the current and former members of the Blackford lab, including Jacqueline Clauss, Ross VanDerKlok, and Brittany Matthews, whose assistance and encouragement have made this work possible. My gratitude goes out to my dissertation committee, who have provided invaluable feedback and whose suggestions have significantly improved this project. Thank you for your insights and help Dr. -

Disrupted Habituation in the Early Stage of Psychosis
Biological Psychiatry: CNNI Archival Report Disrupted Habituation in the Early Stage of Psychosis Suzanne N. Avery, Maureen McHugo, Kristan Armstrong, Jennifer U. Blackford, Neil D. Woodward, and Stephan Heckers ABSTRACT BACKGROUND: Learning and memory are impaired in schizophrenia. Some theories have proposed that one form of memory, habituation, is particularly impaired. Preliminary evidence suggests that memory impairment is associated with failed hippocampal habituation in patients with chronic schizophrenia. We studied how abnormal habituation of the hippocampus is related to relational memory deficits in the early stage of psychosis. METHODS: We measured hippocampal activity in 62 patients with early psychosis and 70 healthy individuals using functional magnetic resonance imaging. Habituation was defined as the slope of functional magnetic resonance imaging signal change to repeated presentations of faces and objects. Relational memory ability was measured as the slope of preferential viewing during a face-scene pair eye movement task outside the scanner. RESULTS: Patients with early psychosis showed impaired relational memory (p , .001) and less hippocampal habituation to objects (p = .01) than healthy control subjects. In the healthy control group, better relational memory was associated with faster anterior hippocampal habituation (faces, r = 2.28, p = .03). In contrast, patients with early psychosis showed no brain-behavior relationship (r = .12, p = .40). CONCLUSIONS: We found evidence for disrupted hippocampal habituation in the early stage of psychosis along with an altered association between hippocampal habituation and relational memory ability. These results suggest that neural habituation may provide a novel target for early cognitive interventions in psychosis. Keywords: First episode, Hippocampus, Novelty, Relational memory, Schizophrenia, Visual cortex https://doi.org/10.1016/j.bpsc.2019.06.007 Repetition is one of the most familiar memory tools. -
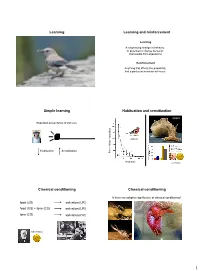
Learning Learning and Reinforcement Simple Learning Habituation and Sensitization Classical Conditioning Classical Conditioning
Learning Learning and reinforcement Learning A long-lasting change in behavior, or potential to change behavior, that results from experience Reinforcement Anything that affects the probability that a particular behavior will occur Simple learning Habituation and sensitization Aplysia Repeated presentation of stimulus chaffinch Habituation Sensitization Percent birds respondingbirds Percent Playbacks Eric Kandel Classical conditioning Classical conditioning Is there any adaptive significance of classical conditioning? food (US) salivation (UR) food (US) + tone (CS) salivation (UR) tone (CS) salivation (CR) Ivan Pavlov 1 Classical conditioning Classical conditioning ♂ ♀ blue gourami Karen Hollis Mount Holyoke Classical conditioning Classical conditioning How might classical conditioning work in nature? Courtship behavior by female (US) Courtship (UR) Courtship behavior by female (US) + sex-recognition cue (CS) Courtship (UR) Sex-recognition cue (CS) Courtship (CR) Japanese quail Heritability of conditioned response Gene × environment interactions Proboscis extension response conditioned to various odors Evidence of significant heritability Tryon Maze Dull and Bright rats Offspring score Offspring Parent score 2 Operant conditioning Laws of effect and exercise Thorndike Operant conditioning Operant conditioning Operant conditioning = association between Negative behavior and its consequences Positive reinforcement reinforcement Increase likelihood that behavior will occur B.F. Skinner Operant conditioning What factors affect conditioning? -

Habituation As a Neural Algorithm for Online Odor Discrimination
Habituation as a neural algorithm for online odor discrimination Yang Shena, Sanjoy Dasguptab, and Saket Navlakhaa,1 aSimons Center for Quantitative Biology, Cold Spring Harbor Laboratory, Cold Spring Harbor, NY 11724; and bComputer Science and Engineering Department, University of California San Diego, La Jolla, CA 92093 Edited by Timothy O’Leary, University of Cambridge, Cambridge, United Kingdom, and accepted by Editorial Board Member Gina G. Turrigiano April 13, 2020 (received for review September 5, 2019) Habituation is a form of simple memory that suppresses neural the extinction of the stimulus. This is in contrast with faster forms activity in response to repeated, neutral stimuli. This process is crit- of habituation, such as odorant receptor neuron (ORN) adapta- ical in helping organisms guide attention toward the most salient tion (17, 18) occurring on the order of hundreds of milliseconds, and novel features in the environment. Here, we follow known and longer forms of habituation, such as long-term habituation circuit mechanisms in the fruit fly olfactory system to derive a (16), which lasts for days. Different time scales of habituation simple algorithm for habituation. We show, both empirically and have also been described in the vertebrate olfactory system (1). analytically, that this algorithm is able to filter out redundant infor- Short-term habituation is of interest because it requires a rela- mation, enhance discrimination between odors that share a similar tively fast online mechanism for its initiation and decay, yet it is background, and improve detection of novel components in odor also stable enough to have a lasting effect. mixtures. Overall, we propose an algorithmic perspective on the Prior work has studied the mechanisms of short-term habitu- biological mechanism of habituation and use this perspective to ation in the mouse and honeybee olfactory circuits (19–22), but understand how sensory physiology can affect odor perception. -

Posttraumatic Stress Disorder DSM IV to 5 Subcategories (4 to 5)
Emory Medicine: Strategic ImplementingRepositioning an Early 2013- 2017Intervention for the Prevention of PTSD in Emergency Department Patients Barbara Olasov Rothbaum, Ph.D., ABPP Paul A. Janssen Chair in Neuropsychopharmacology Director, Trauma and Anxiety Recovery Program Associate Vice Chair of Clinical Research Professor, Department of Psychiatry Emory University School of Medicine Disclosure This study was supported by National Institute of Mental Health Grant Numbers R34 MH083078 and R01 MH071537, and the Emory Center for Injury Control, Center for Disease Control Grant Number 5R49CE001494 and a NARSAD Distinguished Investigator award to Dr. Rothbaum. The authors report no biomedical financial interests or potential conflicts of interest. Recognition and Description of Posttraumatic Stress Disorder DSM IV to 5 subcategories (4 to 5) Criteria DSM-IV DSM-5 A Trauma Trauma B reexperiencing intrusion C Avoidance and avoidance numbing D hyperarousal Negative alterations in cognitions and mood E - Marked alterations in arousal and reactivity A Prospective Examination of PTSD in Rape Victims Percent of Victims with PTS 100 94 80 79 70 64 59 55 60 53 53 45 48 47 41 40 Percent Percent (%) 20 0 1 2 3 4 5 6 7 8 9 10 11 12 Assessment Rothbaum BO et al. J Traumatic Stress. 1992;5(3):463; NIMH Grant No. R01MH42178 Severity of PTSD Symptoms Non-PTS PTS 30 25 20 15 10 5 0 1 2 3 4 5 6 7 8 9 10 11 12 Assessment Rothbaum BO et al. J Traumatic Stress. 1992;5(3):464; NIMH Grant No. R01MH42178 Extinction and Habituation • We view PTSD as a disorder of extinction McSweeney FK, Swindell S. -

'T: Ottawa
csf 001 TOWARD A HUMANISTIC-TRANSPERSONAL PSYCHOLOGY OF RELIGION by Peter A. Campbell Thesis presented to the Department of Religious Studies of the University of Ottawa as partial fulfillment of the requirements for the degree of Doctor of Philosophy ^ «<o% BIBUOTHEQUES * ^^BlBi; 'T: Ottawa LlBRAKicS \ Vsily oi <P Ottawa, Canada, 1972 Peter A. Campbell, Ottawa 1972. UMI Number: DC53628 INFORMATION TO USERS The quality of this reproduction is dependent upon the quality of the copy submitted. Broken or indistinct print, colored or poor quality illustrations and photographs, print bleed-through, substandard margins, and improper alignment can adversely affect reproduction. In the unlikely event that the author did not send a complete manuscript and there are missing pages, these will be noted. Also, if unauthorized copyright material had to be removed, a note will indicate the deletion. UMI® UMI Microform DC53628 Copyright 2011 by ProQuest LLC All rights reserved. This microform edition is protected against unauthorized copying under Title 17, United States Code. ProQuest LLC 789 East Eisenhower Parkway P.O. Box 1346 Ann Arbor, Ml 48106-1346 ACKNOWLEDGMENTS I wish to express my gratitude to Jean-Marie Beniskos, Ph.D., of the Faculty of Education, University of Ottawa, for his helpful direction of this dissertation. My heartfelt thanks are also due to Edwin M. McMahon, my close collabora tor and companion whose constructive criticism and steady encouragement have been a continued source of support through out this research project. CURRICULUM STUDIORUM Peter A. Campbell was born January 21, 1935, in San Francisco, California. He received the Bachelor of Arts degree in 1957 and the Master of Arts degree and Licentiate in Philosophy in 1959 from Gonzaga University, Spokane, Washington. -

The Effect of Habituation and Changes in Cognition on Anxious Children's
Louisiana State University LSU Digital Commons LSU Master's Theses Graduate School 2014 The effect of habituation and changes in cognition on anxious children's performance on the WISC- IV Franziska Noack Louisiana State University and Agricultural and Mechanical College Follow this and additional works at: https://digitalcommons.lsu.edu/gradschool_theses Part of the Psychology Commons Recommended Citation Noack, Franziska, "The effect of habituation and changes in cognition on anxious children's performance on the WISC-IV" (2014). LSU Master's Theses. 988. https://digitalcommons.lsu.edu/gradschool_theses/988 This Thesis is brought to you for free and open access by the Graduate School at LSU Digital Commons. It has been accepted for inclusion in LSU Master's Theses by an authorized graduate school editor of LSU Digital Commons. For more information, please contact [email protected]. THE EFFECT OF HABITUATION AND CHANGES IN COGNITION ON ANXIOUS CHILDREN’S PERFORMANCE ON THE WISC-IV A Thesis Submitted to the Graduate Faculty of the Louisiana State University and Agricultural and Mechanical College in partial fulfillment of the requirements for the degree of Master of Arts in The Department of Psychology by Franziska Noack B.S., University of Houston-Clear Lake, 2012 May 2014 To my husband Daniel NoackLeSage and my parents Ilona and Ralf Noack. Thank you for your continuous support, kind words of encouragement, and loving patience. ------ An meinen Mann Daniel NoackLeSage und meine Eltern, Ilona und Ralf Noack. Danke für eure grenzenlose Unterstützung, eure freundlichen Worte der Ermutigung, und euer liebevolles Verständnis. ii ACKNOWLEDGEMENTS I would like to express my appreciation for the support and time allocated to this project by my advisor and mentor Thompson E.