Additional Tables.Xlsx
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Genome-Wide Analysis of 5-Hmc in the Peripheral Blood of Systemic Lupus Erythematosus Patients Using an Hmedip-Chip
INTERNATIONAL JOURNAL OF MOLECULAR MEDICINE 35: 1467-1479, 2015 Genome-wide analysis of 5-hmC in the peripheral blood of systemic lupus erythematosus patients using an hMeDIP-chip WEIGUO SUI1*, QIUPEI TAN1*, MING YANG1, QIANG YAN1, HUA LIN1, MINGLIN OU1, WEN XUE1, JIEJING CHEN1, TONGXIANG ZOU1, HUANYUN JING1, LI GUO1, CUIHUI CAO1, YUFENG SUN1, ZHENZHEN CUI1 and YONG DAI2 1Guangxi Key Laboratory of Metabolic Diseases Research, Central Laboratory of Guilin 181st Hospital, Guilin, Guangxi 541002; 2Clinical Medical Research Center, the Second Clinical Medical College of Jinan University (Shenzhen People's Hospital), Shenzhen, Guangdong 518020, P.R. China Received July 9, 2014; Accepted February 27, 2015 DOI: 10.3892/ijmm.2015.2149 Abstract. Systemic lupus erythematosus (SLE) is a chronic, Introduction potentially fatal systemic autoimmune disease characterized by the production of autoantibodies against a wide range Systemic lupus erythematosus (SLE) is a typical systemic auto- of self-antigens. To investigate the role of the 5-hmC DNA immune disease, involving diffuse connective tissues (1) and modification with regard to the onset of SLE, we compared is characterized by immune inflammation. SLE has a complex the levels 5-hmC between SLE patients and normal controls. pathogenesis (2), involving genetic, immunologic and envi- Whole blood was obtained from patients, and genomic DNA ronmental factors. Thus, it may result in damage to multiple was extracted. Using the hMeDIP-chip analysis and valida- tissues and organs, especially the kidneys (3). SLE arises from tion by quantitative RT-PCR (RT-qPCR), we identified the a combination of heritable and environmental influences. differentially hydroxymethylated regions that are associated Epigenetics, the study of changes in gene expression with SLE. -

Crispra Screening with Real World Evidence Identifies Potassium Channels As Neuronal Entry Factors and Druggable Targets for SARS-Cov-2
bioRxiv preprint doi: https://doi.org/10.1101/2021.07.01.450475; this version posted July 1, 2021. The copyright holder for this preprint (which was not certified by peer review) is the author/funder. All rights reserved. No reuse allowed without permission. CRISPRa screening with real world evidence identifies potassium channels as neuronal entry factors and druggable targets for SARS-CoV-2 Authors: Chengkun Wang1,†, Ravi K. Dinesh1,†,*, Yuanhao Qu1,2,3,†, Arjun Rustagi4,†, Henry Cousins1,5,‡, James Zengel6,‡, Yinglong Guo7,‡, Taryn Hall7,‡, Aimee Beck4, Luke Tso7, EliF Tokar ErdemiC7, Kae Tanudtanud7, Sheng Ren7, Kathy Tzy-Hwa Tzeng7, Aaron Wilk4,5, Mengdi Wang8, Jan Carette2,6, Russ Altman2,4,9,*, Catherine A. Blish4,5,10,*, Le Cong1,2,3,* Affiliations: 1Department oF Pathology, StanFord University School oF Medicine, StanFord, CA, USA 2Department oF Genetics, StanFord University School oF Medicine, StanFord, CA, USA 3Cancer Biology Program, StanFord University School oF Medicine, StanFord, CA, USA 4Department oF Medicine, StanFord University School oF Medicine, StanFord, CA, USA 5Medical Scientist Training Program, StanFord University School oF Medicine, StanFord, CA, USA 6Department oF Microbiology and Immunology, StanFord University School oF Medicine, StanFord, CA, USA 7Research and Development at UnitedHealth Group, Minneapolis, MN, USA 8Center For Statistics and Machine Learning, Department oF Electrical and Computer Engineering, Princeton University, Princeton, NJ, USA 9Department oF Bioengineering, StanFord University, StanFord, CA, USA 10Chan Zuckerberg Biohub, San Francisco, CA, USA *Correspondence to: [email protected] (R.K.D.); [email protected] (R.A.); [email protected] (C.A.B.); [email protected] (L.C.) † These authors contributed equally to this work. -

Potassium Channels in Epilepsy
Downloaded from http://perspectivesinmedicine.cshlp.org/ on September 28, 2021 - Published by Cold Spring Harbor Laboratory Press Potassium Channels in Epilepsy Ru¨diger Ko¨hling and Jakob Wolfart Oscar Langendorff Institute of Physiology, University of Rostock, Rostock 18057, Germany Correspondence: [email protected] This review attempts to give a concise and up-to-date overview on the role of potassium channels in epilepsies. Their role can be defined from a genetic perspective, focusing on variants and de novo mutations identified in genetic studies or animal models with targeted, specific mutations in genes coding for a member of the large potassium channel family. In these genetic studies, a demonstrated functional link to hyperexcitability often remains elusive. However, their role can also be defined from a functional perspective, based on dy- namic, aggravating, or adaptive transcriptional and posttranslational alterations. In these cases, it often remains elusive whether the alteration is causal or merely incidental. With 80 potassium channel types, of which 10% are known to be associated with epilepsies (in humans) or a seizure phenotype (in animals), if genetically mutated, a comprehensive review is a challenging endeavor. This goal may seem all the more ambitious once the data on posttranslational alterations, found both in human tissue from epilepsy patients and in chronic or acute animal models, are included. We therefore summarize the literature, and expand only on key findings, particularly regarding functional alterations found in patient brain tissue and chronic animal models. INTRODUCTION TO POTASSIUM evolutionary appearance of voltage-gated so- CHANNELS dium (Nav)andcalcium (Cav)channels, Kchan- nels are further diversified in relation to their otassium (K) channels are related to epilepsy newer function, namely, keeping neuronal exci- Psyndromes on many different levels, ranging tation within limits (Anderson and Greenberg from direct control of neuronal excitability and 2001; Hille 2001). -

A Computational Approach for Defining a Signature of Β-Cell Golgi Stress in Diabetes Mellitus
Page 1 of 781 Diabetes A Computational Approach for Defining a Signature of β-Cell Golgi Stress in Diabetes Mellitus Robert N. Bone1,6,7, Olufunmilola Oyebamiji2, Sayali Talware2, Sharmila Selvaraj2, Preethi Krishnan3,6, Farooq Syed1,6,7, Huanmei Wu2, Carmella Evans-Molina 1,3,4,5,6,7,8* Departments of 1Pediatrics, 3Medicine, 4Anatomy, Cell Biology & Physiology, 5Biochemistry & Molecular Biology, the 6Center for Diabetes & Metabolic Diseases, and the 7Herman B. Wells Center for Pediatric Research, Indiana University School of Medicine, Indianapolis, IN 46202; 2Department of BioHealth Informatics, Indiana University-Purdue University Indianapolis, Indianapolis, IN, 46202; 8Roudebush VA Medical Center, Indianapolis, IN 46202. *Corresponding Author(s): Carmella Evans-Molina, MD, PhD ([email protected]) Indiana University School of Medicine, 635 Barnhill Drive, MS 2031A, Indianapolis, IN 46202, Telephone: (317) 274-4145, Fax (317) 274-4107 Running Title: Golgi Stress Response in Diabetes Word Count: 4358 Number of Figures: 6 Keywords: Golgi apparatus stress, Islets, β cell, Type 1 diabetes, Type 2 diabetes 1 Diabetes Publish Ahead of Print, published online August 20, 2020 Diabetes Page 2 of 781 ABSTRACT The Golgi apparatus (GA) is an important site of insulin processing and granule maturation, but whether GA organelle dysfunction and GA stress are present in the diabetic β-cell has not been tested. We utilized an informatics-based approach to develop a transcriptional signature of β-cell GA stress using existing RNA sequencing and microarray datasets generated using human islets from donors with diabetes and islets where type 1(T1D) and type 2 diabetes (T2D) had been modeled ex vivo. To narrow our results to GA-specific genes, we applied a filter set of 1,030 genes accepted as GA associated. -

Supplementary Table 1: Adhesion Genes Data Set
Supplementary Table 1: Adhesion genes data set PROBE Entrez Gene ID Celera Gene ID Gene_Symbol Gene_Name 160832 1 hCG201364.3 A1BG alpha-1-B glycoprotein 223658 1 hCG201364.3 A1BG alpha-1-B glycoprotein 212988 102 hCG40040.3 ADAM10 ADAM metallopeptidase domain 10 133411 4185 hCG28232.2 ADAM11 ADAM metallopeptidase domain 11 110695 8038 hCG40937.4 ADAM12 ADAM metallopeptidase domain 12 (meltrin alpha) 195222 8038 hCG40937.4 ADAM12 ADAM metallopeptidase domain 12 (meltrin alpha) 165344 8751 hCG20021.3 ADAM15 ADAM metallopeptidase domain 15 (metargidin) 189065 6868 null ADAM17 ADAM metallopeptidase domain 17 (tumor necrosis factor, alpha, converting enzyme) 108119 8728 hCG15398.4 ADAM19 ADAM metallopeptidase domain 19 (meltrin beta) 117763 8748 hCG20675.3 ADAM20 ADAM metallopeptidase domain 20 126448 8747 hCG1785634.2 ADAM21 ADAM metallopeptidase domain 21 208981 8747 hCG1785634.2|hCG2042897 ADAM21 ADAM metallopeptidase domain 21 180903 53616 hCG17212.4 ADAM22 ADAM metallopeptidase domain 22 177272 8745 hCG1811623.1 ADAM23 ADAM metallopeptidase domain 23 102384 10863 hCG1818505.1 ADAM28 ADAM metallopeptidase domain 28 119968 11086 hCG1786734.2 ADAM29 ADAM metallopeptidase domain 29 205542 11085 hCG1997196.1 ADAM30 ADAM metallopeptidase domain 30 148417 80332 hCG39255.4 ADAM33 ADAM metallopeptidase domain 33 140492 8756 hCG1789002.2 ADAM7 ADAM metallopeptidase domain 7 122603 101 hCG1816947.1 ADAM8 ADAM metallopeptidase domain 8 183965 8754 hCG1996391 ADAM9 ADAM metallopeptidase domain 9 (meltrin gamma) 129974 27299 hCG15447.3 ADAMDEC1 ADAM-like, -

Single-Cell RNA Sequencing Reveals the Mesangial Identity and Species Diversity of Glomerular Cell Transcriptomes
ARTICLE https://doi.org/10.1038/s41467-021-22331-9 OPEN Single-cell RNA sequencing reveals the mesangial identity and species diversity of glomerular cell transcriptomes Bing He 1,11, Ping Chen 1,11, Sonia Zambrano 1,2, Dina Dabaghie1,2, Yizhou Hu 3, Katja Möller-Hackbarth1, David Unnersjö-Jess 4,5, Gül Gizem Korkut1, Emmanuelle Charrin1,2, Marie Jeansson 1, Maria Bintanel-Morcillo1,2, Anna Witasp6, Lars Wennberg7, Annika Wernerson6, Bernhard Schermer4,5, Thomas Benzing 4,5, Patrik Ernfors 3, Christer Betsholtz 1,8, Mark Lal 9, Rickard Sandberg 10 & ✉ Jaakko Patrakka 1,2 1234567890():,; Molecular characterization of the individual cell types in human kidney as well as model organisms are critical in defining organ function and understanding translational aspects of biomedical research. Previous studies have uncovered gene expression profiles of several kidney glomerular cell types, however, important cells, including mesangial (MCs) and glo- merular parietal epithelial cells (PECs), are missing or incompletely described, and a sys- tematic comparison between mouse and human kidney is lacking. To this end, we use Smart- seq2 to profile 4332 individual glomerulus-associated cells isolated from human living donor renal biopsies and mouse kidney. The analysis reveals genetic programs for all four glo- merular cell types (podocytes, glomerular endothelial cells, MCs and PECs) as well as rare glomerulus-associated macula densa cells. Importantly, we detect heterogeneity in glomerulus-associated Pdgfrb-expressing cells, including bona fide intraglomerular MCs with the functionally active phagocytic molecular machinery, as well as a unique mural cell type located in the central stalk region of the glomerulus tuft. Furthermore, we observe remarkable species differences in the individual gene expression profiles of defined glomerular cell types that highlight translational challenges in the field and provide a guide to design translational studies. -

Accumulation of Neurofascin at Nodes of Ranvier Is Regulated by a Paranodal Switch
The Journal of Neuroscience, July 22, 2020 • 40(30):5709–5723 • 5709 Cellular/Molecular Accumulation of Neurofascin at Nodes of Ranvier Is RegulatedbyaParanodalSwitch Yanqing Zhang,1* Stephanie Yuen,1 Elior Peles,3 and James L. Salzer1,2* 1Neuroscience Institute and Departments of Neuroscience and Physiology and, 2Neurology, New York University School of Medicine, New York, New York 10016, and 3Department of Molecular Cell Biology, Weizmann Institute of Science, Rehovot, 76100, Israel The paranodal junctions flank mature nodes of Ranvier and provide a barrier between ion channels at the nodes and juxta- paranodes. These junctions also promote node assembly and maintenance by mechanisms that are poorly understood. Here, we examine their role in the accumulation of NF186, a key adhesion molecule of PNS and CNS nodes. We previously showed that NF186 is initially targeted/accumulates via its ectodomain to forming PNS (hemi)nodes by diffusion trapping, whereas it is later targeted to mature nodes by a transport-dependent mechanism mediated by its cytoplasmic segment. To address the role of the paranodes in this switch, we compared accumulation of NF186 ectodomain and cytoplasmic domain constructs in WT versus paranode defective (i.e., Caspr-null) mice. Both pathways are affected in the paranodal mutants. In the PNS of Caspr-null mice, diffusion trapping mediated by the NF186 ectodomain aberrantly persists into adulthood, whereas the cyto- plasmic domain/transport-dependent targeting is impaired. In contrast, accumulation of NF186 at CNS nodes does not undergo a switch; it is predominantly targeted to both forming and mature CNS nodes via its cytoplasmic domain and requires intact paranodes. -
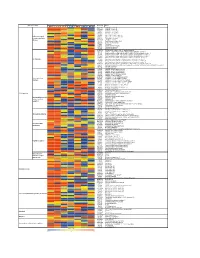
Supporting Figure 2
Normalized value Functional class Genbank Name Naive SC 1h SC 6h SC 24h WM 1h WM 6h WM 24h AB016161 GABA-B receptor 1d AF109405 GABA-B receptor 2a M35077 Dopamine-1A receptor S46131 Dopamine-1A receptor M84009 Dopamine receptor D4 U13368 Adrenergic receptor, alpha 1a G Protein-coupled M60654 Adrenergic receptor, alpha 1d receptors and their M64236 Tachykinin 1 receptor effectors AI229237 Opioid receptor-like Y11433 Pyrimidinergic receptor P2Y4 M64299 Adenosine A1 receptor E00001 Pro-insulin M29014 Insulin receptor precursor U35315 Serotonin receptor 2C S62043 Serotonin receptor 6 AF000368 Scn9a sodium channel, type IX, alpha polypeptide M27158 Kcna5 K+ voltage-gated channel, shaker-related subfamily, member 5 X17621 Kcna6 potassium voltage-gated channel, shaker-related, subfamily, member 6 X16476 Kcnb1 potassium voltage gated channel, Shab-related subfamily, member 1 M77482 Kcnb2 potassium voltage gated channel, Shab-related subfamily, member 2 S64320 Kcnd2 potassium voltage gated channel, Shal-related family, member 2 Ion channels X87635 Kcnj4 potassium inwardly-rectifying channel, subfamily J, member 4 D86039 Kcnj11 potassium inwardly-rectifying channel, subfamily J, member 11 X83581 Kcnj16 potassium inwardly-rectifying channel, subfamily J, member 16 AF073891 Kcnh5 potassium voltage-gated channel, subfamily H (eag-related), member 5 U69882 Kcnn2 potassium intermediate/small conductance calcium-activated channel, subfamily N, member 2 Z36944 Chloride channel 4-2 Z56277 Chloride channel 5 L08493 GABA-A receptor alpha-4 subunit X51992 -

G Protein-Coupled Receptors
www.aladdin-e.com Address:800 S Wineville Avenue, Ontario, CA 91761,USA Website:www.aladdin-e.com Email USA: [email protected] Email EU: [email protected] Email Asia Pacific: [email protected] G PROTEIN-COUPLED RECEPTORS Overview: The completion of the Human Genome Project allowed the identification of a large family of proteins with a common motif of seven groups of 20–24 hydrophobic amino acids arranged as a-helices. Approximately 800 of these seven transmembrane (7TM) receptors have been identified of which over 300 are non-olfactory receptors (see Fredriksson et al., 2003; Lagerstrom and Schioth, 2008). Subdivision on the basis of sequence homology allows the definition of rhodopsin, secretin, adhesion, glutamate and Frizzled receptor families. NC-IUPHAR recognizes Classes A, B, and C, which equate to the rhodopsin, secretin, and glutamate receptor families. The nomenclature of 7TM receptors is commonly used interchangeably with G protein-coupled receptors (GPCR), although the former nomenclature recognises signalling of 7TM receptors through pathways not involving G proteins. For example, adiponectin and membrane progestin receptors have some sequence homology to 7TM receptors but signal independently of G proteins and appear to reside in membranes in an inverted fashion compared to conventional GPCR. Additionally, the NPR-C natriuretic peptide receptor (see Page S195) has a single transmembrane domain structure, but appears to couple to G proteins to generate cellular responses. The 300+ non-olfactory GPCR are the targets for the majority of drugs in clinical usage (Overington et al., 2006), although only a minority of these receptors are exploited therapeutically. -

Changes in Excitability and Ion Channel Expression in Neurons of the Major 2 Pelvic Ganglion in Female Type II Diabetic Mice
bioRxiv preprint doi: https://doi.org/10.1101/360826; this version posted July 4, 2018. The copyright holder for this preprint (which was not certified by peer review) is the author/funder, who has granted bioRxiv a license to display the preprint in perpetuity. It is made available under aCC-BY-NC-ND 4.0 International license. 1 Changes in excitability and ion channel expression in neurons of the major 2 pelvic ganglion in female type II diabetic mice 3 4 5 Michael Gray1*, Kawasi M. Lett1*, Virginia B. Garcia1, Cindy Kyi1, Kathleen A. Pennington2, Laura C. 6 Schulz2, David J. Schulz1 7 8 1 Division of Biological Sciences, University of Missouri, Columbia, MO USA 65211 9 2 Department of Obstetrics, Gynecology and Women's Health, University of Missouri, Columbia, MO, 10 65211, USA. 11 * Denotes equal contribution by these authors 12 13 14 15 Abbreviated Title 16 Changes in parasympathetic neurons in Type II diabetes 17 18 Corresponding Author 19 David J. Schulz, Ph.D. 20 Division of Biological Sciences 21 University of Missouri-Columbia 22 218 LeFevre Hall 23 Columbia, MO 65211 24 Ph 573-882-4067 25 Fax 573-884-5020 26 Email: [email protected] 27 28 29 Acknowledgments 30 This work was funded by a grant from the Missouri Spinal Cord Injuries Research Program (D.J.S.), the 31 Craig H. Neilsen Foundation (D.J.S.), American Diabetes Association Grant 1-14-BS-181 (L.C.S.) and 32 American Heart Association Postdoctoral Fellowship 13POST16910108 (K.A.P.). The authors declare no 33 competing financial interests. -

Gene Expression Evidence for Remodeling of Lateral Hypothalamic Circuitry in Cocaine Addiction
Gene expression evidence for remodeling of lateral hypothalamic circuitry in cocaine addiction Serge H. Ahmed*†‡, Robert Lutjens*‡§, Lena D. van der Stap*, Dusan Lekic*, Vincenzo Romano-Spica¶, Marisela Moralesʈ, George F. Koob*, Vez Repunte-Canonigo*, and Pietro Paolo Sanna*,** *Department of Neuropharmacology, The Scripps Research Institute, La Jolla, CA 92103; †Laboratoire de Neuropsychobiologie des Desadaptations, Universite´Victor Segalen Bordeaux 2, Centre National de la Recherche Scientifique, Unite´Mixte de Recherche 5541, 33076 Bordeaux, France; ¶Dipartmento di Scienze della Salute, Instituto Universitario di Scienze Motorie, Piazza Lauro De Bosis 15, 00194 Rome, Italy; and ʈBehavioral Neuroscience Branch, Intramural Research Program, National Institute on Drug Abuse, National Institutes of Health, 5500 Nathan Shock Drive, Baltimore, MD 21224 Communicated by Floyd E. Bloom, The Scripps Research Institute, La Jolla, CA, May 27, 2005 (received for review December 28, 2004) By using high-density oligonucleotide arrays, we profiled gene model may provide unique clues to the molecular mechanisms expression in reward-related brain regions of rats that developed behind the reward dysfunction that drives the transition to escalated cocaine intake after extended access to cocaine (6 h per compulsive cocaine use. day). Rats allowed restricted daily access to cocaine (only 1 h) that By using high-density oligonucleotide arrays, we profiled gene displayed a stable level of cocaine intake and cocaine naive rats expression changes in several reward-related brain regions in LgA were used for controls. Four analysis methods were compared: rats in comparison with ShA rats and cocaine naive controls. Affymetrix MICROARRAY SUITE 4 and MICROARRAY SUITE 5, which use Results were extensively validated by RT-PCR in individual animals perfect-match-minus-mismatch models, and DCHIP and RMA, which from an independent replication of the experiment. -

G Protein-Coupled Receptors
G PROTEIN-COUPLED RECEPTORS Overview:- The completion of the Human Genome Project allowed the identification of a large family of proteins with a common motif of seven groups of 20-24 hydrophobic amino acids arranged as α-helices. Approximately 800 of these seven transmembrane (7TM) receptors have been identified of which over 300 are non-olfactory receptors (see Frederikson et al., 2003; Lagerstrom and Schioth, 2008). Subdivision on the basis of sequence homology allows the definition of rhodopsin, secretin, adhesion, glutamate and Frizzled receptor families. NC-IUPHAR recognizes Classes A, B, and C, which equate to the rhodopsin, secretin, and glutamate receptor families. The nomenclature of 7TM receptors is commonly used interchangeably with G protein-coupled receptors (GPCR), although the former nomenclature recognises signalling of 7TM receptors through pathways not involving G proteins. For example, adiponectin and membrane progestin receptors have some sequence homology to 7TM receptors but signal independently of G-proteins and appear to reside in membranes in an inverted fashion compared to conventional GPCR. Additionally, the NPR-C natriuretic peptide receptor has a single transmembrane domain structure, but appears to couple to G proteins to generate cellular responses. The 300+ non-olfactory GPCR are the targets for the majority of drugs in clinical usage (Overington et al., 2006), although only a minority of these receptors are exploited therapeutically. Signalling through GPCR is enacted by the activation of heterotrimeric GTP-binding proteins (G proteins), made up of α, β and γ subunits, where the α and βγ subunits are responsible for signalling. The α subunit (tabulated below) allows definition of one series of signalling cascades and allows grouping of GPCRs to suggest common cellular, tissue and behavioural responses.