Carboxylic Acid Derivates
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

(12) United States Patent (10) Patent No.: US 9,642,912 B2 Kisak Et Al
USOO9642912B2 (12) United States Patent (10) Patent No.: US 9,642,912 B2 Kisak et al. (45) Date of Patent: *May 9, 2017 (54) TOPICAL FORMULATIONS FOR TREATING (58) Field of Classification Search SKIN CONDITIONS CPC ...................................................... A61K 31f S7 (71) Applicant: Crescita Therapeutics Inc., USPC .......................................................... 514/171 Mississauga (CA) See application file for complete search history. (72) Inventors: Edward T. Kisak, San Diego, CA (56) References Cited (US); John M. Newsam, La Jolla, CA (US); Dominic King-Smith, San Diego, U.S. PATENT DOCUMENTS CA (US); Pankaj Karande, Troy, NY (US); Samir Mitragotri, Santa Barbara, 5,602,183 A 2f1997 Martin et al. CA (US); Wade A. Hull, Kaysville, UT 5,648,380 A 7, 1997 Martin 5,874.479 A 2, 1999 Martin (US); Ngoc Truc-ChiVo, Longueuil 6,328,979 B1 12/2001 Yamashita et al. (CA) 7,001,592 B1 2/2006 Traynor et al. 7,795,309 B2 9/2010 Kisak et al. (73) Assignee: Crescita Therapeutics Inc., 8,343,962 B2 1/2013 Kisak et al. Mississauga (CA) 8,513,304 B2 8, 2013 Kisak et al. 8,535,692 B2 9/2013 Pongpeerapat et al. (*) Notice: Subject to any disclaimer, the term of this 9,308,181 B2* 4/2016 Kisak ..................... A61K 47/12 patent is extended or adjusted under 35 2002fOOO6435 A1 1/2002 Samuels et al. 2002fOO64524 A1 5, 2002 Cevc U.S.C. 154(b) by 204 days. 2005, OO 14823 A1 1/2005 Soderlund et al. This patent is Subject to a terminal dis 2005.00754O7 A1 4/2005 Tamarkin et al. -

Pharmaceutical Appendix to the Tariff Schedule 2
Harmonized Tariff Schedule of the United States (2007) (Rev. 2) Annotated for Statistical Reporting Purposes PHARMACEUTICAL APPENDIX TO THE HARMONIZED TARIFF SCHEDULE Harmonized Tariff Schedule of the United States (2007) (Rev. 2) Annotated for Statistical Reporting Purposes PHARMACEUTICAL APPENDIX TO THE TARIFF SCHEDULE 2 Table 1. This table enumerates products described by International Non-proprietary Names (INN) which shall be entered free of duty under general note 13 to the tariff schedule. The Chemical Abstracts Service (CAS) registry numbers also set forth in this table are included to assist in the identification of the products concerned. For purposes of the tariff schedule, any references to a product enumerated in this table includes such product by whatever name known. ABACAVIR 136470-78-5 ACIDUM LIDADRONICUM 63132-38-7 ABAFUNGIN 129639-79-8 ACIDUM SALCAPROZICUM 183990-46-7 ABAMECTIN 65195-55-3 ACIDUM SALCLOBUZICUM 387825-03-8 ABANOQUIL 90402-40-7 ACIFRAN 72420-38-3 ABAPERIDONUM 183849-43-6 ACIPIMOX 51037-30-0 ABARELIX 183552-38-7 ACITAZANOLAST 114607-46-4 ABATACEPTUM 332348-12-6 ACITEMATE 101197-99-3 ABCIXIMAB 143653-53-6 ACITRETIN 55079-83-9 ABECARNIL 111841-85-1 ACIVICIN 42228-92-2 ABETIMUSUM 167362-48-3 ACLANTATE 39633-62-0 ABIRATERONE 154229-19-3 ACLARUBICIN 57576-44-0 ABITESARTAN 137882-98-5 ACLATONIUM NAPADISILATE 55077-30-0 ABLUKAST 96566-25-5 ACODAZOLE 79152-85-5 ABRINEURINUM 178535-93-8 ACOLBIFENUM 182167-02-8 ABUNIDAZOLE 91017-58-2 ACONIAZIDE 13410-86-1 ACADESINE 2627-69-2 ACOTIAMIDUM 185106-16-5 ACAMPROSATE 77337-76-9 -

The Influence of Prolonged Cimetidine Administration on Serum Gastrin Levels and Gastric Acid Secretion in Rats
Physiol Res. 41:467-469, 1992 SHORT COMMUNICATION The Influence of Prolonged Cimetidine Administration on Serum Gastrin Levels and Gastric Acid Secretion in Rats A. KOHÚT, O. MAHELOVÁ Department of Pharmacology, Faculty of Medicine, Šafárik University, Kosice Received July 8, 1992 Accepted October 12, 1992 Summary The correlation between serum gastrin levels and gastric acid secretion during 4 weeks of cimetidine administration (once daily) was investigated. Serum gastrin levels and gastric acid secretion were estimated on the 7th, 14th, 21st and 28th day after cimetidine administration (25 mg.kg'1, intragastrically). At the mentioned time intervals gastric acid secretion stimulated by histamine and pentagastrin was also studied. It was found that on the 14th and 21th day after cimetidine administration serum gastrin levels were significantly elevated. Basal gastric acid secretion after cimetidine administration was significantly decreased at all the observed time intervals. Histamine-stimulated gastric acid secretion was increased on the 14th, 21st and 28th day after cimetidine administration Hypoacidity was not followed at all time intervals by hypergastrinaemia (only on day 14 and 21 after cimetidine). Key words Cimetidine - Gastrin concentration - Gastric acid output H2-receptor antagonists (cimetidine, (SIGMA) was given orally (through a gastric tube) in a ranitidine, famotidine) which are potent inhibitors of single dose of 25 mg.kg'1 (dissolved in 0.5 % gastric acid secretion, have proved to be an effective methylcellulose, pH 9.0 - prepared with NaOH). form of treatment of gastric ulceration. It is known that For estimation of the serum gastrin levels and inhibition of acid secretion raises the pH in the gastric gastric acid secretion rats which had fasted for 24 h antrum. -

Cytochrome P450s and Other Enzymes in Drug Metabolism and Toxicity Submitted: December 15 , 2005 ; Accepted: January 12 , 2006 ; Published: March 10, 2006 F
The AAPS Journal 2006; 8 (1) Article 12 (http://www.aapsj.org). Themed Issue: NIDA/AAPS Symposium on Drugs of Abuse: Mechanisms of Toxicity, Toxicokinetics and Medical Consequences, November 4-5, 2005 Guest Editor - Rao S. Rapaka Cytochrome P450s and Other Enzymes in Drug Metabolism and Toxicity Submitted: December 15 , 2005 ; Accepted: January 12 , 2006 ; Published: March 10, 2006 F. Peter Guengerich1 1 Department of Biochemistry and Center in Molecular Toxicology, Vanderbilt University School of Medicine, Nashville, TN 37232 A BSTRACT C HALLENGE OF METABOLISM IN DRUG The cytochrome P450 (P450) enzymes are the major cata- DEVELOPMENT lysts involved in the metabolism of drugs. Bioavailability The numbers related to success in producing new medicines and toxicity are 2 of the most common barriers in drug devel- are very challenging. Even with some discrimination in the opment today, and P450 and the conjugation enzymes can selection of chemicals for leads, ~104 compounds are tested infl uence these effects. The toxicity of drugs can be con- to produce one compound that reaches the market. The aver- sidered in 5 contexts: on-target toxicity, hypersensitivity age cost of taking a drug to the market stage is $800 million and immunological reactions, off-target pharmacology, bio- to $1 billion, and only 1 in 3 drugs that does reach the mar- activation to reactive intermediates, and idiosyncratic drug ket is profi table. Compounding the problems are social and reactions. The chemistry of bioactivation is reasonably well political pressures to both reduce the costs of drugs and pro- understood, but the mechanisms underlying biological re - vide nearly absolute safety. -

Rebound Intragastric Hyperacidity After Abrupt Withdrawal of Histamine
Gut, 1991,32, 1455-1460 1455 Rebound intragastric hyperacidity after abrupt withdrawal ofhistamine H2 receptor blockade Gut: first published as 10.1136/gut.32.12.1455 on 1 December 1991. Downloaded from C U Nwokolo, J T L Smith, A M Sawyerr, R E Pounder Abstract an extensive research programme investigating In a series of 24 hour studies, intragastric the phenomenon of tolerance during continued acidity and plasma gastrin concentration were H2 blockade.'2 '4 measured simultaneously in 46 healthy sub- jects before, during, and 24 to 48 hours after abrupt withdrawal of a histamine H2 receptor Methods antagonist regimen. For 34 days subjects were given either cimetidine 800 mg at night (n=8), SUBJECTS AND DRUG REGIMENS ranitidine 150 mg twice daily (n= 10), ranitidine Forty six of48 healthy men completed the study. 300 mg at night (n= 12), nizatidine 300 mg at Their median age was 21 years (range 19 to 24 night (n=8), or famotidine 40 mg at night years), median weight was 74 kg (range 60 to 96 (n=8). All subjects responded to H2 blockade kg), and median height was 1'80 m (range 1.67 to by a decrease in 24 hour intragastric acidity. 1-93 m). Twenty four smoked cigarettes (4-20 Withdrawal ofH2 blockade resulted in a signifi- cigarettes per day). None had been dosed with an cant rise in median nocturnal integrated intra- antisecretory drug for at least eight weeks before gastric acidity in 42 of46 subjects (+36%; 95% entry to this study. CI +19, +55%) compared with prestudy This study was 'open label,' and all the H2 values, but this rise was not associated with a blockers were supplied by the pharmacy of the significant change in the median integrated Royal Free Hospital, London. -
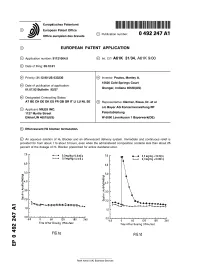
Effervescent H2 Blocker Formulation
Europaisches Patentamt European Patent Office © Publication number: 0 492 247 A1 Office europeen des brevets EUROPEAN PATENT APPLICATION © Application number: 91121064.9 © int. CIA A61K 31/34, A61K 9/00 @ Date of filing: 09.12.91 ® Priority: 21.12.90 US 633230 @ Inventor: Paulos, Manley A. 15626 Cold Springs Court @ Date of publication of application: Indiana 01.07.92 Bulletin 92/27 Granger, 46530(US) © Designated Contracting States: AT BE CH DE DK ES FR GB GR IT LI LU NL SE © Representative: Danner, Klaus, Dr. et al c/o Bayer AG Konzernverwaltung RP © Applicant: MILES INC. 1127 Myrtle Street Patentabteilung ElkhartJN 4651 5(US) W-5090 Leverkusen 1 Bayerwerk(DE) (§v Effervescent H2 blocker formulation. © An aqueous solution of H2 Blocker and an effervescent delivery system. Immediate and continuous relief is provided for from about 1 to about 3 hours, even when the administered composition contains less than about 25 percent of the dosage of H2 Blocker prescribed for active duodenal ulcer. 7.0 r 0,3mg/kg+0,0mEq 7.0 0,3 mg/kg + 32mEq 0,0mg/kg+0,0mEq 0,0mg/kg +0,0mE q 6.0 6,0 5,0 5,0 Xa. *4.0 :4.0 ^3.0 :3,0 1^2,0 h is 2,0 1.0 h 1.0 ■ 0.0 _i i ■ ■ 0.0 CM -60 0 60 120 180 240 -60 0 60 120 180 240 Time After Dosing (Minutes) CM Time After Dosing (Minutes) Oi FIG.1Q FIG.Id Rank Xerox (UK) Business Services EP 0 492 247 A1 Background of the Invention Histamine H2 receptor blocking agents (referred to herein as H2 Blockers) are a class of drugs which act as antagonists of the histamine H2 receptor. -

Ranitidine Protocol – Final Confidential Version 1.0 – 17Th January 2020 PASS Information
RANITIDINE AND OTHER HISTAMINE-H2-RECEPTOR ANTAGONISTS – A DRUG UTILISATION STUDY Principal Investigators Katia Verhamme, MD, PhD Department of Medical Informatics EMC – Rotterdam Peter Rijnbeek, PhD Department of Medical Informatics EMC - Rotterdam Document Status Final Version 1.0 Date of final version of the study 17th January 2020 report EU PAS register number 1 EMA TENDER – Ranitidine protocol – Final Confidential Version 1.0 – 17th January 2020 PASS information Title Ranitidine and other histamine H2-receptor antagonists – a drug utilisation study Protocol version identifier V1.0 Final Date of last version of protocol 2020-01-17 EU PAS register number Active Ingredient Ranitidine Cimetidine Famotidine Nizatidine Niperotidine Roxatidine Ranitidine bismuth citrate Lafutidine Medicinal product Ranitidine Product reference Procedure number Marketing authorisation holder(s) Joint PASS Research question and objectives Ranitidine is a competitive and reversible inhibitor of the action of histamine and indicated for the management of peptic ulceration, Gastro-Esophageal Reflux Disease (GERD), reflux oesophagitis and Zollinger-Ellison syndrome. Results of a preliminary laboratory analysis have shown the presence of N-Nitrosodimethylamine (NDMA), a human carcinogen, in ranitidine. With this DUS, we aim to determine drug utilisation and prescription patterns of medicinal products containing H2-receptor antagonists. These data will give insight on the number of patients using ranitidine and thus potentially at risk of NDMA. In particular we will: 1. Study the prevalence and incidence prescribing of H2-receptor antagonists as a class and by individual drug 2 EMA TENDER – Ranitidine protocol – Final Confidential Version 1.0 – 17th January 2020 2. Explore the characteristics of H2-receptor antagonist use with regard to age (10-years age categories), sex, formulation, daily dose, duration and cumulative exposure by class level and individual ingredient 3. -

(12) Patent Application Publication (10) Pub. No.: US 2002/0102215 A1 100 Ol
US 2002O102215A1 (19) United States (12) Patent Application Publication (10) Pub. No.: US 2002/0102215 A1 Klaveness et al. (43) Pub. Date: Aug. 1, 2002 (54) DIAGNOSTIC/THERAPEUTICAGENTS (60) Provisional application No. 60/049.264, filed on Jun. 6, 1997. Provisional application No. 60/049,265, filed (75) Inventors: Jo Klaveness, Oslo (NO); Pal on Jun. 6, 1997. Provisional application No. 60/049, Rongved, Oslo (NO); Anders Hogset, 268, filed on Jun. 7, 1997. Oslo (NO); Helge Tolleshaug, Oslo (NO); Anne Naevestad, Oslo (NO); (30) Foreign Application Priority Data Halldis Hellebust, Oslo (NO); Lars Hoff, Oslo (NO); Alan Cuthbertson, Oct. 28, 1996 (GB)......................................... 9622.366.4 Oslo (NO); Dagfinn Lovhaug, Oslo Oct. 28, 1996 (GB). ... 96223672 (NO); Magne Solbakken, Oslo (NO) Oct. 28, 1996 (GB). 9622368.0 Jan. 15, 1997 (GB). ... 97OO699.3 Correspondence Address: Apr. 24, 1997 (GB). ... 9708265.5 BACON & THOMAS, PLLC Jun. 6, 1997 (GB). ... 9711842.6 4th Floor Jun. 6, 1997 (GB)......................................... 97.11846.7 625 Slaters Lane Alexandria, VA 22314-1176 (US) Publication Classification (73) Assignee: NYCOMED IMAGING AS (51) Int. Cl." .......................... A61K 49/00; A61K 48/00 (52) U.S. Cl. ............................................. 424/9.52; 514/44 (21) Appl. No.: 09/765,614 (22) Filed: Jan. 22, 2001 (57) ABSTRACT Related U.S. Application Data Targetable diagnostic and/or therapeutically active agents, (63) Continuation of application No. 08/960,054, filed on e.g. ultrasound contrast agents, having reporters comprising Oct. 29, 1997, now patented, which is a continuation gas-filled microbubbles stabilized by monolayers of film in-part of application No. 08/958,993, filed on Oct. -

Federal Register / Vol. 60, No. 80 / Wednesday, April 26, 1995 / Notices DIX to the HTSUS—Continued
20558 Federal Register / Vol. 60, No. 80 / Wednesday, April 26, 1995 / Notices DEPARMENT OF THE TREASURY Services, U.S. Customs Service, 1301 TABLE 1.ÐPHARMACEUTICAL APPEN- Constitution Avenue NW, Washington, DIX TO THE HTSUSÐContinued Customs Service D.C. 20229 at (202) 927±1060. CAS No. Pharmaceutical [T.D. 95±33] Dated: April 14, 1995. 52±78±8 ..................... NORETHANDROLONE. A. W. Tennant, 52±86±8 ..................... HALOPERIDOL. Pharmaceutical Tables 1 and 3 of the Director, Office of Laboratories and Scientific 52±88±0 ..................... ATROPINE METHONITRATE. HTSUS 52±90±4 ..................... CYSTEINE. Services. 53±03±2 ..................... PREDNISONE. 53±06±5 ..................... CORTISONE. AGENCY: Customs Service, Department TABLE 1.ÐPHARMACEUTICAL 53±10±1 ..................... HYDROXYDIONE SODIUM SUCCI- of the Treasury. NATE. APPENDIX TO THE HTSUS 53±16±7 ..................... ESTRONE. ACTION: Listing of the products found in 53±18±9 ..................... BIETASERPINE. Table 1 and Table 3 of the CAS No. Pharmaceutical 53±19±0 ..................... MITOTANE. 53±31±6 ..................... MEDIBAZINE. Pharmaceutical Appendix to the N/A ............................. ACTAGARDIN. 53±33±8 ..................... PARAMETHASONE. Harmonized Tariff Schedule of the N/A ............................. ARDACIN. 53±34±9 ..................... FLUPREDNISOLONE. N/A ............................. BICIROMAB. 53±39±4 ..................... OXANDROLONE. United States of America in Chemical N/A ............................. CELUCLORAL. 53±43±0 -

2 12/ 35 74Al
(12) INTERNATIONAL APPLICATION PUBLISHED UNDER THE PATENT COOPERATION TREATY (PCT) (19) World Intellectual Property Organization International Bureau (10) International Publication Number (43) International Publication Date 22 March 2012 (22.03.2012) 2 12/ 35 74 Al (51) International Patent Classification: (81) Designated States (unless otherwise indicated, for every A61K 9/16 (2006.01) A61K 9/51 (2006.01) kind of national protection available): AE, AG, AL, AM, A61K 9/14 (2006.01) AO, AT, AU, AZ, BA, BB, BG, BH, BR, BW, BY, BZ, CA, CH, CL, CN, CO, CR, CU, CZ, DE, DK, DM, DO, (21) International Application Number: DZ, EC, EE, EG, ES, FI, GB, GD, GE, GH, GM, GT, PCT/EP201 1/065959 HN, HR, HU, ID, IL, IN, IS, JP, KE, KG, KM, KN, KP, (22) International Filing Date: KR, KZ, LA, LC, LK, LR, LS, LT, LU, LY, MA, MD, 14 September 201 1 (14.09.201 1) ME, MG, MK, MN, MW, MX, MY, MZ, NA, NG, NI, NO, NZ, OM, PE, PG, PH, PL, PT, QA, RO, RS, RU, (25) Filing Language: English RW, SC, SD, SE, SG, SK, SL, SM, ST, SV, SY, TH, TJ, (26) Publication Language: English TM, TN, TR, TT, TZ, UA, UG, US, UZ, VC, VN, ZA, ZM, ZW. (30) Priority Data: 61/382,653 14 September 2010 (14.09.2010) US (84) Designated States (unless otherwise indicated, for every kind of regional protection available): ARIPO (BW, GH, (71) Applicant (for all designated States except US): GM, KE, LR, LS, MW, MZ, NA, SD, SL, SZ, TZ, UG, NANOLOGICA AB [SE/SE]; P.O Box 8182, S-104 20 ZM, ZW), Eurasian (AM, AZ, BY, KG, KZ, MD, RU, TJ, Stockholm (SE). -

Niperotidine Hydrochloride
Metopimazine/Nizatidine 1751 ataxia, confusion, disorientation, dizziness, euphoria, dysphoria, little effect on the metabolism of other drugs. However, hallucinations, psychosis, depression, headache, decreased con- like other H2-antagonists its effects on gastric pH may centration, blurred vision, sleep disturbances, decreased coordi- O affect the absorption of some other drugs. nation, and tremors. Adverse cardiovascular reactions including -O N+ hypotension, orthostatic hypotension, and tachycardia have oc- curred. Gastrointestinal disturbances, decreased appetite, and ab- HN Pharmacokinetics dominal pain have also been reported. Nizatidine is readily and almost completely absorbed O HN Precautions S from the gastrointestinal tract. The bioavailability of Nabilone is extensively metabolised and largely excreted in bile, O CH3 nizatidine after oral doses exceeds 70% and may be and therefore is not recommended in patients with severe hepatic O N slightly increased by the presence of food. It is widely impairment. It should be used with caution in patients with a his- CH3 distributed and is about 35% bound to plasma proteins. tory of psychiatric disorders or depression, or those with hyper- tension or heart disease. (niperotidine) The elimination half-life of nizatidine is 1 to 2 hours Because of the possibility of CNS depression, patients should be and is prolonged in renal impairment. Nizatidine is warned not to drive or operate machinery. Profile partly metabolised in the liver: nizatidine N-2-oxide, Niperotidine hydrochloride is a histamine H2-receptor antagonist nizatidine S-oxide, and N-2-monodesmethylnizatidine The possibility of dependence similar to that of cannabis should with general properties similar to those of cimetidine (p.1716). be borne in mind. -

Histamine Receptor
Histamine Receptor Histamine Receptors are a class of G protein-coupled receptors with histamine as their endogenous ligand. There are four known histamine receptors: H1 receptor, H2 receptor, H3 receptor, H4 receptor. The H1 receptor is a histamine receptor belonging to the family of Rhodopsin-like G-protein-coupled receptors. This receptor, which is activated by the biogenic amine histamine, is expressed throughout the body, to be specific, in smooth muscles, on vascular endothelial cells, in the heart, and in the central nervous system. H2 receptors are positively coupled to adenylate cyclase via Gs. It is a potent stimulant of cAMP production, which leads to activation of Protein Kinase A. Histamine H3 receptors are expressed in the central nervous system and to a lesser extent the peripheral nervous system, where they act asautoreceptors in presynaptic histaminergic neurons, and also control histamine turnover by feedback inhibition of histamine synthesis and release. The Histamine H4 receptor has been shown to be involved in mediating eosinophil shape change and mast cell chemotaxis. www.MedChemExpress.com 1 Histamine Receptor Inhibitors & Modulators (±)-Methotrimeprazine (D6) (±)-Tazifylline (dl-Methotrimeprazine D6) Cat. No.: HY-19489S Cat. No.: HY-U00018 Bioactivity: (±)-Methotrimeprazine (D6) is the deuterium labeled Bioactivity: (±)-Tazifylline is a potent, selective and long-acting Methotrimeprazine, which is a D3 dopamine and Histamine H1 histamine H1 receptor antagonist. receptor antagonist. Purity: >98% Purity: >98% Clinical Data: No Development Reported Clinical Data: No Development Reported Size: 1 mg Size: 1 mg, 5 mg, 10 mg, 20 mg ABT-239 Acrivastine Cat. No.: HY-12195 (BW825C) Cat. No.: HY-B1510 Bioactivity: ABT-239 is a novel, highly efficacious, Bioactivity: Acrivastine (BW825C) is a short acting histamine 1 non-imidazole class of H3R antagonist and a transient receptor antagonist for the treatment of allergic rhinitis.