Rebound Intragastric Hyperacidity After Abrupt Withdrawal of Histamine
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Carboxylic Acid Derivates
Europaisches Patentamt 0 367 484 J> European Patent Office CO Publication number: A1 Office europeen des brevets © EUROPEAN PATENT APPLICATION A61K 31/29 © Application number: 89310994.2 © int. ci.5: C07D 233/64 , C07D 277/42 C07D 277/28 @ Date of filing: 25.10.89 C07D 295/08 C07D 249/14 C07D 211/20 C07D 417/04 © Priority: 26.10.88 GB 8825058 © Applicant: GLAXO GROUP LIMITED 26.06.89 GB 8914631 Clarges House 6-12 Clarges Street London W1Y8DH(GB) © Date of publication of application: 09.05.90 Bulletin 90/19 © Inventor: Clitherow, John Watson 54, Gilders © Designated Contracting States: Sawbridgeworth Hertfordshire(GB) AT BE CH DE ES FR GB GR IT LI LU NL SE © Representative: Marchant, James Ian et al Elkington and Fife Beacon House 113 Kingsway London WC2B 6PP(GB) © Carboxylic acid derivates. © The invention relates to salts formed between basic H2-receptor antagonists and a complex of bismuth with a carboxylic acid, and solvate of such salts, excluding salts in which the basic H2-receptor antagonist is ranitidine. Examples of suitable carboxylic acids are citric acid and tartaric acid. Examples of basic rVreceptor antagonists are cimetidine, sufotidine famotidine and nizatidine. The salts ares useful in the treatment of gastrointestinal disorders, particularly gastroduodenal conditions. The salts show the antisecretory activity associated with the basic H2-receptor antagonist together with antibacterial activity against Campylobacter pylori and they also possess cytoprotective properties. 00 (0 a. LU Xerox Copy Centre EP 0 367 484 A1 CARBOXYLIC ACID DERIVATIVES This invention relates to salts of compounds having antagonist activity at histamine H2- receptors, to a process for the preparation thereof, to pharmaceutical compositions containing them and to their use in therapeutics. -

Pharmacology and Toxicology of Metiamide, a Histamine H
9 November 1974 S.-A. MEDIESE TYDSKRIF 2253 Pharmacology and Toxicology of Metiamide, a - Histamine H2 Receptor Antagonist R. W. BRIMBLECOMBE, W. A. M. DUNCAN, M. E. PARSONS SUMMARY x 10-'M on atrial muscle and 7,5 x lO-rM on uterine muscle. Even. at lO-'M, metiamide did not inhibit the A brief review of the pharmacology and toxicology of effects of isoprenaline on either of these tissues neither did it inhibit the effects of histamine on isolated Quinea- metiamide, a histamine H2-receptor antagonist, is given, ~ and evidence is presented to support the view that it pig ileum (mediated through H,-receptors). inhibits gastric acid secretion by virtue of its Hrreceptor Metiamide is also an inhibitor of gastric acid secretion. antagonist activity. Its effectiveness was estimated in two preparations: the Studies are also reported which show that metiamide lumen-perfused stomach of the anaesthetised rat' and given either intravenously or intraduodenally inhibits his the conscious Heidenhain pouch dog, prepared 1 - 3 years tamine- or pentagastrin-stimulated acid secretion in human before experimentation. Metiamide was given by rapid subjects. llltravenous injection during a maximal plateau of acid secretion stimulated by either histamine or pentagastrin, and the dose required to reduce this level of secretion by S. Afr. Med. J., 48, 2253 (1974). 50% (ED",) was estimated. The results are shown in Table I and indicate that the ED", values are very similar Conventional antihistaminic drugs, such as mepyramine. even in high concentrations, fail to inhibit histamine against those of both histamine and pentagastrin. Doses of stimulated gastric acid secretion. -

United States Patent (19) (11) 4,310,524 Wiech Et Al
United States Patent (19) (11) 4,310,524 Wiech et al. 45 Jan. 12, 1982 (54) TCA COMPOSITION AND METHOD FOR McMillen et al., Fed. Proc., 38,592 (1979). RAPD ONSET ANTDEPRESSANT Sellinger et al., Fed. Proc., 38,592 (1979). THERAPY Pandey et al., Fed. Proc., 38,592 (1979). 75) Inventors: Norbert L. Wiech; Richard C. Ursillo, Primary Examiner-Stanley J. Friedman both of Cincinnati, Ohio Attorney, Agent, or Firm-Millen & White 73) Assignee: Richardson-Merrell, Inc., Wilton, Conn. (57 ABSTRACT A method is provided for treating depression in a pa (21) Appl. No.: 139,498 tient therefrom and requiring rapid symptomatic relief, (22 Filed: Apr. 11, 1980 which comprises administering to said patient concur 51) Int. Cl. .................... A61K 31/33; A61K 31/135 rently (a) an effective antidepressant amount of a tricy clic antidepressant or a pharmaceutically effective acid (52) ...... 424/244; 424/330 addition salt thereof, and (b) an amount of an a-adrener 58) Field of Search ................................ 424/244, 330 gic receptor blocking agent effective to achieve rapid (56) References Cited onset of the antidepressant action of (a), whereby the PUBLICATIONS onset of said antidepressant action is achieved within Chemical Abst., vol. 66-72828m, (1967), Kellett. from 1 to 7 days. Chemical Abst, vol. 68-94371a, (1968), Martelli et al. A pharmaceutical composition is also provided which is Chemical Abst., vol. 74-86.048j, (1971), Dixit et al. especially adapted for use with the foregoing method. Holmberg et al., Psychopharm., 2,93 (1961). Svensson, Symp. Med. Hoechst., 13, 245 (1978). 17 Claims, No Drawings 4,310,524 1. -

(12) United States Patent (10) Patent No.: US 9,642,912 B2 Kisak Et Al
USOO9642912B2 (12) United States Patent (10) Patent No.: US 9,642,912 B2 Kisak et al. (45) Date of Patent: *May 9, 2017 (54) TOPICAL FORMULATIONS FOR TREATING (58) Field of Classification Search SKIN CONDITIONS CPC ...................................................... A61K 31f S7 (71) Applicant: Crescita Therapeutics Inc., USPC .......................................................... 514/171 Mississauga (CA) See application file for complete search history. (72) Inventors: Edward T. Kisak, San Diego, CA (56) References Cited (US); John M. Newsam, La Jolla, CA (US); Dominic King-Smith, San Diego, U.S. PATENT DOCUMENTS CA (US); Pankaj Karande, Troy, NY (US); Samir Mitragotri, Santa Barbara, 5,602,183 A 2f1997 Martin et al. CA (US); Wade A. Hull, Kaysville, UT 5,648,380 A 7, 1997 Martin 5,874.479 A 2, 1999 Martin (US); Ngoc Truc-ChiVo, Longueuil 6,328,979 B1 12/2001 Yamashita et al. (CA) 7,001,592 B1 2/2006 Traynor et al. 7,795,309 B2 9/2010 Kisak et al. (73) Assignee: Crescita Therapeutics Inc., 8,343,962 B2 1/2013 Kisak et al. Mississauga (CA) 8,513,304 B2 8, 2013 Kisak et al. 8,535,692 B2 9/2013 Pongpeerapat et al. (*) Notice: Subject to any disclaimer, the term of this 9,308,181 B2* 4/2016 Kisak ..................... A61K 47/12 patent is extended or adjusted under 35 2002fOOO6435 A1 1/2002 Samuels et al. 2002fOO64524 A1 5, 2002 Cevc U.S.C. 154(b) by 204 days. 2005, OO 14823 A1 1/2005 Soderlund et al. This patent is Subject to a terminal dis 2005.00754O7 A1 4/2005 Tamarkin et al. -

Antagonism of Histamine-Activated Adenylate Cyclase in Brain by D
Proc. Natl. Acad. Sci. USA Vol.74, No. 12, pp. 5697-5701, December 1977 Medical Sciences Antagonism of histamine-activated adenylate cyclase in brain by D-lysergic acid diethylamide (histaminergic antagonists/adenosine 3':5'-cyclic monophosphate/H2-receptors/ergots/D-2-bromolysergic acid diethylamide) JACK PETER GREEN, CARL LYNN JOHNSON, HAREL WEINSTEIN, AND SAUL MAAYANI Department of Pharmacology, Mount Sinai School of Medicine of the City University of New York, 100th Street and Fifth Avenue, New York, New- York 10029 Communicated by Vincent P. Dole, August 19, 1977 ABSTRACT D-Lysergic acid diethylamide and D-2-bro- (ED50; amount necessary to produce half-maximal response) molysergic acid diethylamide are competitive antagonists of and antagonist affinities (pA2) were not altered. the histamine activation of adenylate cyclase [ATP pyrophos- Adenylate Cyclase Assay. The assay system has been de- phate-lyase (cyclizing); E.C. 4.6.1.11 in broken cell preparations in All additions of the hippocampus and cortex of guinea pig brain. The ade- scribed (8). All assays were performed triplicate. nylate cyclase is linked to the histamine H2-receptor. Both D- were made to the assay tubes on ice. They were then transferred lysergic acid diethylamide and D-2-bromolysergic acid dieth- to a 30° shaking incubator and preincubated for 5 min to allow ylamide show topological congruency with potent H2-antago- the enzymatic activity to reach a steady state and to eliminate nists. D-2-Bromolysergic acid diethylamide is 10 times more the influence of any lag periods in hormone activation. After potent as an H2-antagonist than cimetidine, which has been the the preincubation period, 25 of [a-32PJATP (1-2 gCi) were most potent H2-antagonist reported, and D-lysergic acid di- pl ethylamide is about equipotent to cimetidine. -

The Influence of Prolonged Cimetidine Administration on Serum Gastrin Levels and Gastric Acid Secretion in Rats
Physiol Res. 41:467-469, 1992 SHORT COMMUNICATION The Influence of Prolonged Cimetidine Administration on Serum Gastrin Levels and Gastric Acid Secretion in Rats A. KOHÚT, O. MAHELOVÁ Department of Pharmacology, Faculty of Medicine, Šafárik University, Kosice Received July 8, 1992 Accepted October 12, 1992 Summary The correlation between serum gastrin levels and gastric acid secretion during 4 weeks of cimetidine administration (once daily) was investigated. Serum gastrin levels and gastric acid secretion were estimated on the 7th, 14th, 21st and 28th day after cimetidine administration (25 mg.kg'1, intragastrically). At the mentioned time intervals gastric acid secretion stimulated by histamine and pentagastrin was also studied. It was found that on the 14th and 21th day after cimetidine administration serum gastrin levels were significantly elevated. Basal gastric acid secretion after cimetidine administration was significantly decreased at all the observed time intervals. Histamine-stimulated gastric acid secretion was increased on the 14th, 21st and 28th day after cimetidine administration Hypoacidity was not followed at all time intervals by hypergastrinaemia (only on day 14 and 21 after cimetidine). Key words Cimetidine - Gastrin concentration - Gastric acid output H2-receptor antagonists (cimetidine, (SIGMA) was given orally (through a gastric tube) in a ranitidine, famotidine) which are potent inhibitors of single dose of 25 mg.kg'1 (dissolved in 0.5 % gastric acid secretion, have proved to be an effective methylcellulose, pH 9.0 - prepared with NaOH). form of treatment of gastric ulceration. It is known that For estimation of the serum gastrin levels and inhibition of acid secretion raises the pH in the gastric gastric acid secretion rats which had fasted for 24 h antrum. -

Drug and Medication Classification Schedule
KENTUCKY HORSE RACING COMMISSION UNIFORM DRUG, MEDICATION, AND SUBSTANCE CLASSIFICATION SCHEDULE KHRC 8-020-1 (11/2018) Class A drugs, medications, and substances are those (1) that have the highest potential to influence performance in the equine athlete, regardless of their approval by the United States Food and Drug Administration, or (2) that lack approval by the United States Food and Drug Administration but have pharmacologic effects similar to certain Class B drugs, medications, or substances that are approved by the United States Food and Drug Administration. Acecarbromal Bolasterone Cimaterol Divalproex Fluanisone Acetophenazine Boldione Citalopram Dixyrazine Fludiazepam Adinazolam Brimondine Cllibucaine Donepezil Flunitrazepam Alcuronium Bromazepam Clobazam Dopamine Fluopromazine Alfentanil Bromfenac Clocapramine Doxacurium Fluoresone Almotriptan Bromisovalum Clomethiazole Doxapram Fluoxetine Alphaprodine Bromocriptine Clomipramine Doxazosin Flupenthixol Alpidem Bromperidol Clonazepam Doxefazepam Flupirtine Alprazolam Brotizolam Clorazepate Doxepin Flurazepam Alprenolol Bufexamac Clormecaine Droperidol Fluspirilene Althesin Bupivacaine Clostebol Duloxetine Flutoprazepam Aminorex Buprenorphine Clothiapine Eletriptan Fluvoxamine Amisulpride Buspirone Clotiazepam Enalapril Formebolone Amitriptyline Bupropion Cloxazolam Enciprazine Fosinopril Amobarbital Butabartital Clozapine Endorphins Furzabol Amoxapine Butacaine Cobratoxin Enkephalins Galantamine Amperozide Butalbital Cocaine Ephedrine Gallamine Amphetamine Butanilicaine Codeine -

Further Analysis of Anomalous Pkbvalues for Histamine
Br. J. Pharmac. (1985), 86, 581-587 Further analysis ofanomalous pKB values for histamine H2-receptor antagonists on the mouse isolated stomach assay J.W. Blackk, P. Leff* & N.P. Shankley The Rayne Institute, King's College Hospital Medical School, Denmark Hill, London SE5 8RX and Wellcome Research Laboratories*, Langley Court, Beckenham, Kent, BR3 3BS 1 Agonist-antagonist interactions at histamine receptors have been re-examined using improved techniques, on the mouse isolated, lumen-perfused, stomach gastric acid assay. 2 Using histamine as agonist, pKB values have been estimated for burimamide, metiamide, cimetidine, ranitidine, oxmetidine and famotidine on both the gastric and guinea-pig isolated right atrium assays. With the exception of oxmetidine on the atrial assay, these compounds behaved as competitive antagonists on both assays. 3 Oxmetidine significantly depressed basal rate on the atrial assay and the Schild plot slope parameter (0.81) was significantly less than one. 4 The pKB values estimated on the gastric assay were lower than those on the atrial assay. However, the difference between the values on the gastric and atrial assays was not constant. The difference between the two assays for famotidine was not significant. 5 We conclude that the apparent varying selectivity ofthe antagonists for gastric and atrial histamine H2-receptors may be explained by the differential loss ofantagonists into the gastric secretion from the receptor compartment and that there is no need to postulate heterogeneity ofhistamine H2-receptors. Introduction Angus & Black (1979) and Angus et al. (1980) found Pearce (1981) did not find a low pKB for metiamide that the histamine H2-receptor antagonists, using the rat isolated gastric mucosa preparation for burimamide, metiamide and cimetidine behaved as the assay. -

Platelets by Burimamide G
Br. J. Pharmac. (1980), 71, 157-164 INHIBITION OF THROMBOXANE A2 BIOSYNTHESIS IN HUMAN PLATELETS BY BURIMAMIDE G. ALLAN, K.E. EAKINS*, P.S. KULKARNI* & R. LEVI Department of Pharmacology, Cornell University Medical College, New York, N.Y. and *Departments of Opthalmology and Pharmacology, Columbia University College of Physicians & Surgeons, New York, N.Y., U.S.A. 1 Burimamide selectively inhibited the formation of thromboxane A2 from prostaglandin endoper- oxides by human platelet microsomes in a dose-dependent manner (IC50 = 2.5 x 10-5 M). Burima- mide was found to be equipotent to imidazole as a thromboxane synthetase inhibitor. 2 Metiamide, cimetidine and a series of compounds either bearing a structural or pharmacological relationship to histamine caused little or no inhibition of thromboxane A2 biosynthesis by human platelet microsomes. 3 Burimamide (5 x 10-4 to 2.3 x 10- M) did not inhibit either the cyclo-oxygenase or the prosta- cyclin synthetase of sheep seminal vesicles or the prostacyclin synthetase of dog aortic microsomes. 4 Burimamide (2.5 x 10'- to 1.2 x 10-4 M) inhibited sodium arachidonate-induced human platelet aggregation; the degree of inhibition was dependent upon the concentration of arachidonic acid used to aggregate the platelets. Introduction The prostaglandin endoperoxides (prostaglandin G2, (Gryglewski, Zmuda, Korbut, Krecioch & Bieron, H2), the first cyclo-oxygenated products of arachido- 1977). nic acid metabolism can be converted enzymatically Various imidazole derivatives were studied by into either primary prostaglandins such as prosta- Moncada et al. (1977) and of these, only one-methyl glandin E2, F2, or D2, the non-prostanoate throm- imidazole was found to be a potent inhibitor of boxane A2 or prostacyclin (Moncada, Gryglewski, thromboxane A2 biosynthesis. -

Selective and Competitive Histamine H2-Receptor Blocking Effect of Famotidine on the Blood Pressure Response in Dogs and the Acid Secretory Response in Rats
Selective and Competitive Histamine H2-Receptor Blocking Effect of Famotidine on the Blood Pressure Response in Dogs and the Acid Secretory Response in Rats Keiji MIYATA, Takeshi KAMATO, Akira FUJIHARA and Masaaki TAKEDA Department of Pharmacology, Medicinal Research Laboratories I, Yamanouchi Pharmaceutical Co., Ltd., 21 Miyukigaoka, Tsukuba , Ibaraki 305, Japan Accepted July 17, 1990 Abstract-Famotidine has been already demonstrated to be a competitive H2- receptor antagonist in the stomachs of dogs and cats. The present experiments were carried out to examine the effects of famotidine on changes in blood pressure induced by dimaprit and several other agonists in vagotomized, anesthetized dogs and on changes in gastric acid secretion induced by histamine in stomach-perfused, anesthetized rats. Famotidine caused a parallel displacement of the dimaprit dose-response curve to the right with a DR10 value of 0.059 ƒÊmol/kg, indicating that famotidine is 166 times more potent than cimetidine in vascular H2-blocking activity. On the contrary, famotidine did not affect the depressor responses to 2- pyridylethylamine and histamine that were antagonized by mepyramine. The histamine dose-response curve was displaced to the right more markedly after simultaneous administration of mepyramine and famotidine than after mepyramine alone. The effects of methacholine, phenylephrine and isoproterenol on blood pressure were not influenced by famotidine in doses up to 720 nmol/kg. In rats, famotidine also caused a parallel displacement of the acid dose-response curve to histamine to the right with a DR3 value of 24 ƒÊmol/kg/hr in stomach-perfused rats anesthetized with pentobarbital, exhibiting a potency 108 times greater than that of cimetidine. -
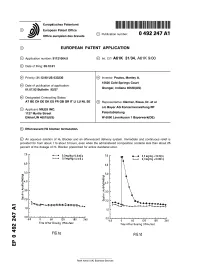
Effervescent H2 Blocker Formulation
Europaisches Patentamt European Patent Office © Publication number: 0 492 247 A1 Office europeen des brevets EUROPEAN PATENT APPLICATION © Application number: 91121064.9 © int. CIA A61K 31/34, A61K 9/00 @ Date of filing: 09.12.91 ® Priority: 21.12.90 US 633230 @ Inventor: Paulos, Manley A. 15626 Cold Springs Court @ Date of publication of application: Indiana 01.07.92 Bulletin 92/27 Granger, 46530(US) © Designated Contracting States: AT BE CH DE DK ES FR GB GR IT LI LU NL SE © Representative: Danner, Klaus, Dr. et al c/o Bayer AG Konzernverwaltung RP © Applicant: MILES INC. 1127 Myrtle Street Patentabteilung ElkhartJN 4651 5(US) W-5090 Leverkusen 1 Bayerwerk(DE) (§v Effervescent H2 blocker formulation. © An aqueous solution of H2 Blocker and an effervescent delivery system. Immediate and continuous relief is provided for from about 1 to about 3 hours, even when the administered composition contains less than about 25 percent of the dosage of H2 Blocker prescribed for active duodenal ulcer. 7.0 r 0,3mg/kg+0,0mEq 7.0 0,3 mg/kg + 32mEq 0,0mg/kg+0,0mEq 0,0mg/kg +0,0mE q 6.0 6,0 5,0 5,0 Xa. *4.0 :4.0 ^3.0 :3,0 1^2,0 h is 2,0 1.0 h 1.0 ■ 0.0 _i i ■ ■ 0.0 CM -60 0 60 120 180 240 -60 0 60 120 180 240 Time After Dosing (Minutes) CM Time After Dosing (Minutes) Oi FIG.1Q FIG.Id Rank Xerox (UK) Business Services EP 0 492 247 A1 Background of the Invention Histamine H2 receptor blocking agents (referred to herein as H2 Blockers) are a class of drugs which act as antagonists of the histamine H2 receptor. -

(12) Patent Application Publication (10) Pub. No.: US 2002/0102215 A1 100 Ol
US 2002O102215A1 (19) United States (12) Patent Application Publication (10) Pub. No.: US 2002/0102215 A1 Klaveness et al. (43) Pub. Date: Aug. 1, 2002 (54) DIAGNOSTIC/THERAPEUTICAGENTS (60) Provisional application No. 60/049.264, filed on Jun. 6, 1997. Provisional application No. 60/049,265, filed (75) Inventors: Jo Klaveness, Oslo (NO); Pal on Jun. 6, 1997. Provisional application No. 60/049, Rongved, Oslo (NO); Anders Hogset, 268, filed on Jun. 7, 1997. Oslo (NO); Helge Tolleshaug, Oslo (NO); Anne Naevestad, Oslo (NO); (30) Foreign Application Priority Data Halldis Hellebust, Oslo (NO); Lars Hoff, Oslo (NO); Alan Cuthbertson, Oct. 28, 1996 (GB)......................................... 9622.366.4 Oslo (NO); Dagfinn Lovhaug, Oslo Oct. 28, 1996 (GB). ... 96223672 (NO); Magne Solbakken, Oslo (NO) Oct. 28, 1996 (GB). 9622368.0 Jan. 15, 1997 (GB). ... 97OO699.3 Correspondence Address: Apr. 24, 1997 (GB). ... 9708265.5 BACON & THOMAS, PLLC Jun. 6, 1997 (GB). ... 9711842.6 4th Floor Jun. 6, 1997 (GB)......................................... 97.11846.7 625 Slaters Lane Alexandria, VA 22314-1176 (US) Publication Classification (73) Assignee: NYCOMED IMAGING AS (51) Int. Cl." .......................... A61K 49/00; A61K 48/00 (52) U.S. Cl. ............................................. 424/9.52; 514/44 (21) Appl. No.: 09/765,614 (22) Filed: Jan. 22, 2001 (57) ABSTRACT Related U.S. Application Data Targetable diagnostic and/or therapeutically active agents, (63) Continuation of application No. 08/960,054, filed on e.g. ultrasound contrast agents, having reporters comprising Oct. 29, 1997, now patented, which is a continuation gas-filled microbubbles stabilized by monolayers of film in-part of application No. 08/958,993, filed on Oct.