Preservation of Marine Products by Salting and Drying
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Influences of Squid Ink Added to Low Salt Fermented Squid on Its Changes in Lactic Acid Bacteria
Korean J. Food & Nutr. Vol. 26. No. 4, 678~684 (2013) THE KOREAN JOURNAL OF http://dx.doi.org/10.9799/ksfan.2013.26.4.678 한국식품영양학회지 FOOD AND NUTRITION Influences of Squid Ink Added to Low Salt Fermented Squid on Its Changes in Lactic Acid Bacteria †Sung-Cheon Oh Dept. of Food & Pharmacy, Daewon University College, Jecheon 390-702, Korea 저염 오징어 젓갈의 숙성 중 오징어 먹즙 첨가가 젖산균의 변화에 미치는 영향 †오 성 천 대원대학교 제약식품계열 국문요약 오징어 먹즙을 첨가하지 않은 저염 오징어 젓갈은 숙성 발효에 따라 숙성 초기에 젖산균 총수가 급속히 증가하고, Leuconostoc이 증가하여 숙성 적기에 최대량을 나타내고 이후 서서히 감소하였다. Lactobacillus는 숙성 적기 이후의 총 균수의 대부분을 차지할 만큼 균수가 많아지므로 숙성 적기 이후 품질에 관여하는 것으로 보인다. Streptococcus와 Pediococcus는 숙성 적기까지 완만하게 증가하다가 이후 급격히 감소하였다. Yeast는 숙성 중기 이후에 검출되고, 숙성 말기에 급격히 증가하므로 부패에 주로 관여하는 것으로 보인다. 오징어 먹즙을 첨가한 저염 오징어 젓갈의 숙성발효에 따른 젖산균의 변화를 보면 총 균수는 숙성 중반까지 증가하다가 중반 이후 약간 감소하였으나, 최대치에 도달하는 기간은 무 첨가군에 비하여 길었고, 젖산균 중 Leuconostoc, Streptococcus 및 Pediococcus는 숙성 후반까지 증가하였다가 후반에 감소하는 반면에, Lactobacillus는 숙성 후반까지 계속 증가하였다. Yeast는 숙성 초기에는 거의 증가하지 않다가 숙성 중반 이후 증가하였는데, 이러한 경향은 무 첨가군과 유사하였으나 젖산균의 수가 무 첨가군에 비하여 적었다. 오징어 먹즙을 첨가한 저염 오징어 젓갈에서 모든 균들의 수가 감소되어 오징어 먹즙이 균들의 성장 을 억제하는 것으로 확인되었다. Key words: lactic acid bacteria, low salt fermented squid, squid ink, yeast, ripening Introduction is a dominant strain in the ripening stage regardless of the salt concentration although higher salt concentration reduces the Fermented squid is a fermented seafood product which contains bacterial count in an extract (Mori et al. -

Thermal Behavior, Microstructure and Protein Quality of Squid Fillets Dried
Food Control 36 (2014) 102e110 Contents lists available at ScienceDirect Food Control journal homepage: www.elsevier.com/locate/foodcont Thermal behavior, microstructure and protein quality of squid fillets dried by far-infrared assisted heat pump drying Yun Deng a, Yuegang Wang a, Jin Yue a,*, Zhenmin Liu b, Yuanrong Zheng b, Bingjun Qian b, Yu Zhong a, Yanyun Zhao a,c,* a SJTU-Bor Luh Food Safety Center, Department of Food Science and Technology, Shanghai Jiao Tong University, 800 Dongchuan Road, Shanghai 200240, PR China b Dairy Research Institute, Bright Dairy & Food Co., Ltd., Bldg 2, No.1518, West Jiangchang Road, Shanghai 200436, PR China c Department of Food Science and Technology, 100 Wiegand Hall, Oregon State University, Corvallis, OR, USA article info abstract Article history: The effects of far-infrared assisted heat pump drying on the thermal behavior, microstructure and protein Received 6 May 2013 quality of squid fillets were studied. Heat pump drying (HPD) alone or in combination with far-infrared À Received in revised form radiation (FIR) at the power of 100, 500 and 800 W, 40 C and 2 m s 1 air velocity were compared. 3 August 2013 Nitrogen fractions, proteases activities, amino acid profile, microstructure, SDS-PAGE and protein quality Accepted 5 August 2013 of fresh and dried squid samples were examined. Results showed that glutamic acid, aspartic acid, lysine and isoleucine are the dominant amino acids in all samples. The total contents of essential amino acid Keywords: and non-essential amino acid, essential amino acid index, estimated protein efficiency ratio and bio- Heat pump drying fi > Far-infrared radiation logical value were not signi cantly different among all squid samples (P 0.05). -
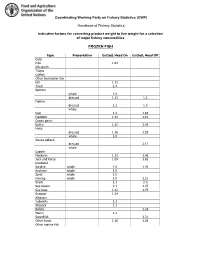
Indicative Factors for Converting Product Weight to Live Weight for a Selection of Major Fishery Commodities
Coordinating Working Party on Fishery Statistics (CWP) Handbook of Fishery Statistics Indicative factors for converting product weight to live weight for a selection of major fishery commodities FROZEN FISH Type Prese tatio Gutted, Head O Gutted, Head Off Carp Pike 1.04 Nile perch Tilapia Catfish Other freshwater fish Eel 1.11 Trout 1.4 Salmon: whole 1.0 dressed 1.1 1. Flatfish: dressed 1.1 1.4 whole Cod 1.2 1.69 Haddock 1.14 1.61 Ocean perch Saithe 1.15 1.45 Hake: dressed 1.16 1.55 whole 1.0 Alaska pollack: dressed 2.17 whole Capelin Mackerel 1.11 1.46 Jack and horse 1.09 1.65 mackerel Sardine whole 1.0 1.4 Anchovy whole 1.0 Sprat whole 1.0 Herring whole 1.0 1.52 Shark 1.1 2.0 Sea .ream 1.1 1.47 Sea .ass 1.12 1.79 Snapper 1.14 Al.acore /ellowfin 1.1 Skip0ack 1.1 1onito 1.29 Marlin 1.1 Swordfish 1. 1 Other tunas 1.16 1. 6 Other marine fish FILLETS Type Prese tatio Raw CF Ski Off CF Flatfish 2.42 2.61 Herring 1.62 2.17 Cod 2.45 .2 fillet .2 portion .55 mince .2 Mackerel 1.95 2.60 Haddock 2.77 2.91 fillet 2.21 portion .20 Ocean perch 2.92 .11 Saithe 2.12 2.55 Hake 2.5 2.90 fillet 2.90 portion .19 mince 2.90 Alaska pollack .72 fillet .72 mince .72 Salmon 2.00 2.00 steaks 1.60 Tuna 1.54 1.92 loins Catfish 2.6 .55 steaks 2.40 FISH DRIED, WHETHER OR NOT SALTED Basic Conversion Factor for Basic Type Deduced CF Product Product Stockfish gutted cod 1.2 1.94 Klipfish filleted cod 2.45 3.97 Hake fillet 2.53 4.1 Shark fillet 2.4 3.89 Shark fins 0 Tuna fillet 1.54 2.49 Pilchard fillet 1.62 2.62 Tilapia gutted 1.2 1.94 Other freshwater fillet 2.45 4 Other fish fillet 2.45 4 N1: the conversion factor for dried fish is taken as 1.62 times the CF for the .asic product FISH SALTED, WET OR IN BRINE Type O,served Co versio Fa.tors Mea or 3range4 Re.omme ded CF Freshwater fish 1.5 Cod 1.79 5 2.2 2.0 Other demersal 1. -

Mycological Evaluation of Smoked-Dried Fish Sold at Maiduguri Metropolis, Nigeria: Preliminary Findings and Potential Health Implications
Original Article / Orijinal Makale doi: 10.5505/eurjhs.2016.69885 Eur J Health Sci 2016;2(1):5-10 Mycological Evaluation of Smoked-Dried Fish Sold at Maiduguri Metropolis, Nigeria: Preliminary Findings and Potential Health Implications Nijerya’da Maiduguri Metropolis’te satılan tütsülenmiş balıkların mikolojik değerlendirilmesi: Ön bulgular ve sağlığa potansiyel etkileri 1 2 3 Fatima Muhammad Sani , Idris Abdullahi Nasir , Gloria Torhile 1Department of Medical Laboratory Science, College of Medical Sciences University of Maiduguri, Borno State, Nigeria 2Department of Medical Microbiology, University of Abuja Teaching Hospital, Gwagwalada, FCT Abuja, Nigeria 3Department of Medical Microbiology, Federal Teaching Hospital Gombe, Gombe, Nigeria ABSTRACT Background: Smoked-dried fish are largely consumed as source of nutrient by man. It has been established that fish food can act as vehicle for transmission of some mycological pathogens especially in immunocompromised individuals. Methods: Between 7th October 2011 and 5th January 2012, a total of 100 different species of smoke-dried fish comprising 20 each of Cat fish (Arius hendeloti), Tilapia (Oreochromis niloticus), Stock fish (Gadus morhua), Mud fish (Neoxhanna galaxiidae) and Bonga fish (Enthalmosa fimbriota) were processed and investigated for possible fungal contamination based on culture isolation using Sabouraud dextrose agar (SDA) and microscopy. Results: Organisms isolated and identified in pure culture were Mucor spp. (36%), Aspergillus niger (35%), Aspergillus fumigatus (6%), Candida tropicalis (3%), Candida stellatoidea (2%), Microsporum audunii (2%), Penicillium spp. (2%), and Trichophyton rubrum (1%) while Mucor spp. and Aspergillus niger (4%); Mucor spp. and Candida tropicalis (3%); Aspergillus fumigatus and Mucor spp. (1%); Aspergillus niger, Candida spp. and Mucor spp. (1%) were isolated in mixed culture. -

A Report on the Sodium Levels of Salted Dry Fish in Chennai And
The Pharma Innovation Journal 2018; 7(11): 01-04 ISSN (E): 2277- 7695 ISSN (P): 2349-8242 NAAS Rating: 5.03 A report on the sodium levels of salted dry fish in TPI 2018; 7(11): 01-04 © 2018 TPI Chennai and recommendations to comply regulation www.thepharmajournal.com Received: 01-09-2018 Accepted: 02-10-2018 A Devi Dharshini, M Latha Mala Priyadharshini, D Baskaran and A Devi Dharshini G Dhinakar Raj College of Food and Dairy Technology, TANUVAS, Abstract Koduveli, Chennai, Tamil Nadu, Regulating the intake of sodium is in need of the hour pertaining to the established health effects of high India sodium consumption. Labeled food products from organized food sectors provide information on sodium M Latha Mala Priyadharshini levels, while in unorganized sector the sodium content of some of the heavily salted food products such Assistant Professor, Vaccine as dry fish goes unnoticed. This report is presented based on the studies from 12 commercial dry fish Research Centre – Bacterial samples collected form Chennai, one of the major sea food export coastal region of India. The samples Vaccine, Centre for Animal were tested for NaCl, sodium and moisture content. The NaCl content was found to be well above 15% in Health Studies, Madhavaram 7 samples, between 10-15% in 2 samples and below 10% in 3 samples. Consequently 9 samples had Milk Colony, Madhavaram, sodium content above 2000 mG/100G and it was evident that NaCl content of more than half the samples Chennai, Tamil Nadu, India was not in compliance with the FSSAI regulations of 10-15% NaCl content by weight of salted dry fish. -

Plant Phenolics: Bioavailability As a Key Determinant of Their Potential Health-Promoting Applications
antioxidants Review Plant Phenolics: Bioavailability as a Key Determinant of Their Potential Health-Promoting Applications Patricia Cosme , Ana B. Rodríguez, Javier Espino * and María Garrido * Neuroimmunophysiology and Chrononutrition Research Group, Department of Physiology, Faculty of Science, University of Extremadura, 06006 Badajoz, Spain; [email protected] (P.C.); [email protected] (A.B.R.) * Correspondence: [email protected] (J.E.); [email protected] (M.G.); Tel.: +34-92-428-9796 (J.E. & M.G.) Received: 22 October 2020; Accepted: 7 December 2020; Published: 12 December 2020 Abstract: Phenolic compounds are secondary metabolites widely spread throughout the plant kingdom that can be categorized as flavonoids and non-flavonoids. Interest in phenolic compounds has dramatically increased during the last decade due to their biological effects and promising therapeutic applications. In this review, we discuss the importance of phenolic compounds’ bioavailability to accomplish their physiological functions, and highlight main factors affecting such parameter throughout metabolism of phenolics, from absorption to excretion. Besides, we give an updated overview of the health benefits of phenolic compounds, which are mainly linked to both their direct (e.g., free-radical scavenging ability) and indirect (e.g., by stimulating activity of antioxidant enzymes) antioxidant properties. Such antioxidant actions reportedly help them to prevent chronic and oxidative stress-related disorders such as cancer, cardiovascular and neurodegenerative diseases, among others. Last, we comment on development of cutting-edge delivery systems intended to improve bioavailability and enhance stability of phenolic compounds in the human body. Keywords: antioxidant activity; bioavailability; flavonoids; health benefits; phenolic compounds 1. Introduction Phenolic compounds are secondary metabolites widely spread throughout the plant kingdom with around 8000 different phenolic structures [1]. -

Consolidated Version of the Sanpin 2.3.2.1078-01 on Food, Raw Material, and Foodstuff
Registered with the Ministry of Justice of the RF, March 22, 2002 No. 3326 MINISTRY OF HEALTH OF THE RUSSIAN FEDERATION CHIEF STATE SANITARY INSPECTOR OF THE RUSSIAN FEDERATION RESOLUTION No. 36 November 14, 2001 ON ENACTMENT OF SANITARY RULES (as amended by Amendments No.1, approved by Resolution No. 27 of Chief State Sanitary Inspector of the RF dated 20.08.2002, Amendments and Additions No. 2, approved by Resolution No. 41 of Chief State Sanitary Inspector of the RF dated15.04.2003, No. 5, approved by Resolution No. 42 of Chief State Sanitary Inspector of the RF dated 25.06.2007, No. 6, approved by Resolution No. 13 of Chief State Sanitary Inspector of the RF dated 18.02.2008, No. 7, approved by Resolution No. 17 of Chief State Sanitary Inspector of the RF dated 05.03.2008, No. 8, approved by Resolution No. 26 of Chief State Sanitary Inspector of the RF dated 21.04.2008, No. 9, approved by Resolution No. 30 of Chief State Sanitary Inspector of the RF dated 23.05.2008, No. 10, approved by Resolution No. 43 of Chief State Sanitary Inspector of the RF dated 16.07.2008, Amendments No.11, approved by Resolution No. 56 of Chief State Sanitary Inspector of the RF dated 01.10.2008, No. 12, approved by Resolution No. 58 of Chief State Sanitary Inspector of the RF dated 10.10.2008, Amendment No. 13, approved by Resolution No. 69 of Chief State Sanitary Inspector of the RF dated 11.12.2008, Amendments No.14, approved by Resolution No. -

Oxidation in Foods and Beverages and Antioxidant Applications
Oxidation in foods and beverages and antioxidant applications ß Woodhead Publishing Limited, 2010 Related titles: Oxidation in foods and beverages and antioxidant applications Volume 2 Management in different industry sectors (ISBN 978-1-84569-983-3) Oxidative rancidity is a major cause of food quality deterioration, leading to the formation of undesirable off-flavours as well as unhealthy compounds. The two volumes of Oxidation in foods and beverages and antioxidant applications review food quality deterioration due to oxidation and methods for its control. The second volume concentrates on oxidation and its management in different industry sectors. Part I discusses animal products, with chapters on the oxidation and protection of red meat, poultry, fish and dairy products. Part II reviews oxidation in plant-based foods and beverages, including edible oils, wine and fried products. The final chapters examine encapsulation to inhibit lipid oxidation and antioxidant active packaging and edible films. Chemical deterioration and physical instability of foods and beverages (ISBN 978-1-84569-495-1) For a food product to be a success in the marketplace, it must be stable throughout its shelf-life. Changes due to food chemical deterioration and physical instability are not always recognised by food producers, who are more familiar with microbial spoilage, yet can be just as problematic. This book provides an authoritative review of key topics in this area. Chapters in Parts I and II focus on the chemical reactions and physical changes that negatively affect food quality. The remaining chapters outline the likely effects on different food products, for example baked goods, fruit and vegetables and beverages. -

Unravel Browning Mechanism in Making Kue Delapan Jam
International Food Research Journal 24(1): 310-317 (February 2017) Journal homepage: http://www.ifrj.upm.edu.my Unravel browning mechanism in making kue delapan jam 1*Agustini, S., 2Priyanto, G., 2Hamzah, B., 2Santoso, B. and 2Pambayun, R. 1Palembang Institute for Industrial Research and Standardization, Jalan Perindustrian 2 nomor 12 Sukarami Palembang, Indonesia 30152 2Department of Agricultural Technology, College of Agriculture, Sriwijaya University, Jl. Padang Selasa Nomor 524 Bukit Besar Palembang, Indonesia 30139 Article history Abstract Received: 14 September 2015 This research intended to study browning reaction mechanism in making kue delapan jam Received in revised form: and its implication on nutritional value. Browning reaction among substance exist in raw 8 March 2016 material during processing influence the sensories quality and color of cake significantly. Accepted: 14 March 2016 The study was based on the theoretical approaches relate to browning reaction, taking into account the experimental test, material balance, and the available information. The reaction pathway (Maillard reaction, caramelization, lipid oxidation) was determined by the changes Keywords in concentration of raw material (dough) and selected observable chemical marker according to their ability to indentify reaction stages. Research applied completely randomized design Browning with steaming time as a treatment (0 h, and 8 hours) with 3 replications. Reaction pathway Kue delapan jam Reaction mechanism determined by observing changes of sucrose, lactose, glucose, fructose, hidroxy methyl furfural, Steaming acetic acid, formic acid, amino acid and fatty acid between the dough and the cake. Test results showed that there were no significant changes in sucrose concentration. No fructose, glucose, acetic acid, formic acid, and hidroxy methyl furfural indentified both in the dough and the cake. -

The Role of Lipids in Nonenzymatic Browning
Grasas y Aceites 35 Vol. 51. Fasc. 1-2 (2000), 35-49 The role of lipids in nonenzymatic browning By Francisco J. Hidalgo and Rosario Zamora Instituto de la Grasa, CSIC, Avenida Padre García Tejero 4, 41012 Sevilla, Spain RESUMEN 1. INTRODUCTION El papel de los lípidos en el pardeamiento no enzimático When we select foods and when we eat, we En este trabajo se hace una revisión del papel de los lípidos en use all of our physical senses, including sight, el pardeamiento no enzimático de alimentos mediante el estudio de touch, smell, taste, and even hearing. These las reacciones proteína/lípido oxidado en comparación con otras senses allow us to determine food quality, which reacciones donde ocurre también este oscurecimiento: la reacción de Maillard, el pardeamiento producido por el ácido ascórbico, y las can be divided into three main categories: reacciones de las quinonas con los grupos amino. Los appearance, textural and flavor factors (Francis, mecanismos propuestos para estas reacciones de producción de 1999; Ghorpade et al. 1995; Potter and Hotchkiss, color y fluorescencia, así como la formación de melanoidinas, 1995). Appearance factors include such things as lipofuscinas y productos coloreados de bajo peso molecular son size, shape, wholeness, different forms of discutidos de forma comparada, concluyendo que el papel de los lípidos en estas reacciones no parece ser muy diferente del papel damage, gloss, transparency, color, and de los carbohidratos en el Maillard o de los fenoles en el par- consistency. Textural factors include handfeel and deamiento enzimático. Estas reacciones carbonil-amino parecen mouthfeel of firmness, softness, juiciness, ser un grupo de reacciones secundarias que ocurren de forma chewiness, grittiness. -

Relevance of Physical Properties in the Stability of Plant-Based Food Products
Indian Journal of Experimental Biology Vol. 51, November 2013, pp. 895-904 Review Article Relevance of physical properties in the stability of plant-based food products Elena Venir* & Enrico Maltini Department of Food Science, University of Udine, Via Sondrio 2/A, 33100 Udine, Italy Plant tissues are composed of a watery solution of low molecular weight species, mainly sugars, salts and organic acids, and of high molecular weight hydrocolloids, contained in a water insoluble matrix of macromolecules, mostly carbohydrates. All these constituents interact with water, thus reducing its thermodynamic vapour pressure (aw), with small molecules interacting through polar binding, and large biopolymers through surface and capillary effects. Similarly, some constituents will greatly affect kinetic glass transition temperatures (Tg), while others will not. As regards stability, while microbial and chemical changes are mainly related to aw, structure-related changes such as collapse are dependent on the glass transition temperature, Tg. In simple systems such as juices, both thermodynamic and kinetic approaches, employed respectively for high and low moisture systems, have predictive ability, which can be unified in the concept of “critical aw”. However, in complex, multidomain, multiphase systems, such as vegetables and fruits, where insoluble polymeric phases are present, hydrocolloids such as soluble pectins will only slightly affect Tg and aw, but significantly increase the macro viscosity of the soluble fraction, thereby reducing the tendency to collapse. In such cases the use of Tg as a predictive tool must be considered with care. The interrelationships among these aspects are discussed in detail below. Keywords: Glass transition, Plant food, Stability, Water activity Food stability is influenced by chemical, physical and hydrocolloids4. -

View Travel Planning Guide
YOUR O.A.T. ADVENTURE TRAVEL PLANNING GUIDE® New! Under the Midnight Sun: Sami Lapland, Norway & the Arctic Circle 2021 Small Groups: 8-16 travelers—guaranteed! (average of 13) Overseas Adventure Travel ® The Leader in Personalized Small Group Adventures on the Road Less Traveled 1 Dear Traveler, At last, the world is opening up again for curious travel lovers like you and me. And the O.A.T. New! Under the Midnight Sun: Sami Lapland, Norway & the Arctic Circle itinerary you’ve expressed interest in will be a wonderful way to resume the discoveries that bring us so much joy. You might soon be enjoying standout moments like these: There was something intangibly magical about Lapland. Maybe it was the midnight sun, the endless rugged tundra, or the welcoming nature of the Sami people. All I know is that there was a true sense of Arctic magic everywhere I went, especially when I met an indigenous Sami family on their reindeer farm. As we explored the farm, they introduced me to their way of life and traditions dating back thousands of years. I was saddened to hear that their ancient culture is under threat from two forces: the construction of an Arctic Railway through Sami territory and Sami youth deviating from their traditional lifestyle. You’ll hear about these challenges as well when you meet with a Sami family on their reindeer farm. In the regions I travel to around the world, the stories of the people who live and work there are the most distinct and poignant experiences. You’ll meet with a local educator in Oslo to hear about July 22, 2011—the harrowing terrorist attack on this city—and their personal account of this day.