Variation in Wood Density Determines Spatial Patterns in Amazonian Forest Biomass
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Annals of the Missouri Botanical Garden 1988
- Annals v,is(i- of the Missouri Botanical Garden 1988 # Volume 75 Number 1 Volume 75, Number ' Spring 1988 The Annals, published quarterly, contains papers, primarily in systematic botany, con- tributed from the Missouri Botanical Garden, St. Louis. Papers originating outside the Garden will also be accepted. Authors should write the Editor for information concerning arrangements for publishing in the ANNALS. Instructions to Authors are printed on the inside back cover of the last issue of each volume. Editorial Committee George K. Rogers Marshall R. Crosby Editor, Missouri B Missouri Botanical Garden Editorial is. \I,,S ouri Botanu •al Garde,, John I). Dwyer Missouri Botanical Garden Saint Louis ( niversity Petei • Goldblatt A/I.S.S ouri Botanic al Garder Henl : van der W< ?rff V//.S.S ouri Botanic tor subscription information contact Department IV A\NM.S OK Tin: Missot m Boi >LM« M G\KDE> Eleven, P.O. Box 299, St. Louis, MO 63166. Sub- (ISSN 0026-6493) is published quarterly by the scription price is $75 per volume U.S., $80 Canada Missouri Botanical Garden, 2345 Tower Grove Av- and Mexico, $90 all other countries. Airmail deliv- enue, St. Louis, MO 63110. Second class postage ery charge, $35 per volume. Four issues per vol- paid at St. Louis, MO and additional mailing offices. POSTMAS'IKK: Send ad«lrt— changes to Department i Botanical Garden 1988 REVISED SYNOPSIS Grady L. Webster2 and Michael J. Huft" OF PANAMANIAN EUPHORBIACEAE1 ABSTRACT species induded in \ • >,H The new taxa ai I. i i " I ! I _- i II • hster, Tragia correi //,-," |1 U !. -

Chec List What Survived from the PLANAFLORO Project
Check List 10(1): 33–45, 2014 © 2014 Check List and Authors Chec List ISSN 1809-127X (available at www.checklist.org.br) Journal of species lists and distribution What survived from the PLANAFLORO Project: PECIES S Angiosperms of Rondônia State, Brazil OF 1* 2 ISTS L Samuel1 UniCarleialversity of Konstanz, and Narcísio Department C.of Biology, Bigio M842, PLZ 78457, Konstanz, Germany. [email protected] 2 Universidade Federal de Rondônia, Campus José Ribeiro Filho, BR 364, Km 9.5, CEP 76801-059. Porto Velho, RO, Brasil. * Corresponding author. E-mail: Abstract: The Rondônia Natural Resources Management Project (PLANAFLORO) was a strategic program developed in partnership between the Brazilian Government and The World Bank in 1992, with the purpose of stimulating the sustainable development and protection of the Amazon in the state of Rondônia. More than a decade after the PLANAFORO program concluded, the aim of the present work is to recover and share the information from the long-abandoned plant collections made during the project’s ecological-economic zoning phase. Most of the material analyzed was sterile, but the fertile voucher specimens recovered are listed here. The material examined represents 378 species in 234 genera and 76 families of angiosperms. Some 8 genera, 68 species, 3 subspecies and 1 variety are new records for Rondônia State. It is our intention that this information will stimulate future studies and contribute to a better understanding and more effective conservation of the plant diversity in the southwestern Amazon of Brazil. Introduction The PLANAFLORO Project funded botanical expeditions In early 1990, Brazilian Amazon was facing remarkably in different areas of the state to inventory arboreal plants high rates of forest conversion (Laurance et al. -

Los Géneros De La Familia Euphorbiaceae En México (Parte D) Anales Del Instituto De Biología
Anales del Instituto de Biología. Serie Botánica ISSN: 0185-254X [email protected] Universidad Nacional Autónoma de México México Martínez Gordillo, Martha; Jiménez Ramírez, Jaime; Cruz Durán, Ramiro; Juárez Arriaga, Edgar; García, Roberto; Cervantes, Angélica; Mejía Hernández, Ricardo Los géneros de la familia Euphorbiaceae en México (parte D) Anales del Instituto de Biología. Serie Botánica, vol. 73, núm. 2, julio-diciembre, 2002, pp. 245-281 Universidad Nacional Autónoma de México Distrito Federal, México Disponible en: http://www.redalyc.org/articulo.oa?id=40073208 Cómo citar el artículo Número completo Sistema de Información Científica Más información del artículo Red de Revistas Científicas de América Latina, el Caribe, España y Portugal Página de la revista en redalyc.org Proyecto académico sin fines de lucro, desarrollado bajo la iniciativa de acceso abierto GÉNEROS DE EUPHORBIACEAE 245 Fig. 42. Hippomane mancinella. A, rama; B, glándula; C, inflorescencia estaminada (Marín G. 75, FCME). 246 M. MARTÍNEZ GORDILLO ET AL. Se reconoce por tener una glándula en la unión de la lámina y el pecíolo, por el haz, el ovario 6-9-locular y los estilos cortos. Tribu Hureae 46. Hura L., Sp. Pl. 1008. 1753. Tipo: Hura crepitans L. Árboles monoicos; corteza con espinas cónicas; exudado claro. Hojas alternas, simples, hojas usualmente ampliamente ovadas y subcordatas, márgenes serrados, haz y envés glabros o pubescentes; nervadura pinnada; pecíolos largos y con dos glándulas redondeadas al ápice; estípulas pareadas, imbricadas, caducas. Inflorescencias unisexuales, glabras, las estaminadas terminales, largo- pedunculadas, espigadas; bractéolas membranáceas; flor pistilada solitaria en las axilas de las hojas distales. Flor estaminada pedicelada, encerrada en una bráctea delgada que se rompe en la antesis; cáliz unido formando una copa denticulada; pétalos ausentes; disco ausente; estambres numerosos, unidos, filamentos ausen- tes, anteras sésiles, verticiladas y lateralmente compresas en 2-10 verticilos; pistilodio ausente. -

MICROPROPAGACIÓN DE PILÓN (Hieronyma Alchorneoides)
Agronomía Costarricense 35(2): 9-19. ISSN:0377-9424 / 2011 www.mag.go.cr/rev agr/index.html www.cia.ucr.ac.cr MICROPROPAGACIÓN DE PILÓN (Hieronyma alchorneoides) Ana Abdelnour1/*, Mª Elena Aguilar**, Lissette Valverde†*** Palabras clave: Micropropagación, cultivo in vitro, pilón, Hieronyma alchorneoides, citocininas. Keywords: Micropropagation, in vitro culture, pilon, Hieronyma alchorneoides, cytokinins. Recibido: 05/07/11 Aceptado: 23/09/11 RESUMEN ABSTRACT El pilón (Hieronyma alchorneoides, Micropropagation of pilon (Hieronyma Euphorbiaceae) es una de las especies nativas alchorneoides). Pilon (Hieronyma alchorneoides) maderables de Costa Rica mejor adaptadas a (Euphorbiaceae) is one of the native timber condiciones abiertas de plantación; por sus carac- species of Costa Rica best adapted to conditions of terísticas de crecimiento y variedad de usos, la open plantation. Due to its growth characteristics demanda por material de siembra se ha incre- and variety of uses, the demand for planting mentado. Esta especie presenta serios problemas material has increased. This species presents en su reproducción sexual: es dioica, la produc- serious problems with seed reproduction; it is ción de frutos muy variable en el tiempo y entre a dioecious, the fruit production varies over árboles, que producen gran cantidad de frutos, time and between trees, trees produce large pero son fuertemente depredados por diferentes quantities of fruit but are heavily predated by aves. En los últimos años el porcentaje de frutos different birds. In recent years, the percentage dañados se ha incrementado a niveles conside- of damaged fruit has increased due to wasps and rables debido a avispas y las semillas pierden su the seeds lose their ability to germinate within capacidad de germinación pocos días después de days of harvest (recalcitrant). -

The One Hundred Tree Species Prioritized for Planting in the Tropics and Subtropics As Indicated by Database Mining
The one hundred tree species prioritized for planting in the tropics and subtropics as indicated by database mining Roeland Kindt, Ian K Dawson, Jens-Peter B Lillesø, Alice Muchugi, Fabio Pedercini, James M Roshetko, Meine van Noordwijk, Lars Graudal, Ramni Jamnadass The one hundred tree species prioritized for planting in the tropics and subtropics as indicated by database mining Roeland Kindt, Ian K Dawson, Jens-Peter B Lillesø, Alice Muchugi, Fabio Pedercini, James M Roshetko, Meine van Noordwijk, Lars Graudal, Ramni Jamnadass LIMITED CIRCULATION Correct citation: Kindt R, Dawson IK, Lillesø J-PB, Muchugi A, Pedercini F, Roshetko JM, van Noordwijk M, Graudal L, Jamnadass R. 2021. The one hundred tree species prioritized for planting in the tropics and subtropics as indicated by database mining. Working Paper No. 312. World Agroforestry, Nairobi, Kenya. DOI http://dx.doi.org/10.5716/WP21001.PDF The titles of the Working Paper Series are intended to disseminate provisional results of agroforestry research and practices and to stimulate feedback from the scientific community. Other World Agroforestry publication series include Technical Manuals, Occasional Papers and the Trees for Change Series. Published by World Agroforestry (ICRAF) PO Box 30677, GPO 00100 Nairobi, Kenya Tel: +254(0)20 7224000, via USA +1 650 833 6645 Fax: +254(0)20 7224001, via USA +1 650 833 6646 Email: [email protected] Website: www.worldagroforestry.org © World Agroforestry 2021 Working Paper No. 312 The views expressed in this publication are those of the authors and not necessarily those of World Agroforestry. Articles appearing in this publication series may be quoted or reproduced without charge, provided the source is acknowledged. -

D-299 Webster, Grady L
UC Davis Special Collections This document represents a preliminary list of the contents of the boxes of this collection. The preliminary list was created for the most part by listing the creators' folder headings. At this time researchers should be aware that we cannot verify exact contents of this collection, but provide this information to assist your research. D-299 Webster, Grady L. Papers. BOX 1 Correspondence Folder 1: Misc. (1954-1955) Folder 2: A (1953-1954) Folder 3: B (1954) Folder 4: C (1954) Folder 5: E, F (1954-1955) Folder 6: H, I, J (1953-1954) Folder 7: K, L (1954) Folder 8: M (1954) Folder 9: N, O (1954) Folder 10: P, Q (1954) Folder 11: R (1954) Folder 12: S (1954) Folder 13: T, U, V (1954) Folder 14: W (1954) Folder 15: Y, Z (1954) Folder 16: Misc. (1949-1954) D-299 Copyright ©2014 Regents of the University of California 1 Folder 17: Misc. (1952) Folder 18: A (1952) Folder 19: B (1952) Folder 20: C (1952) Folder 21: E, F (1952) Folder 22: H, I, J (1952) Folder 23: K, L (1952) Folder 24: M (1952) Folder 25: N, O (1952) Folder 26: P, Q (1952-1953) Folder 27: R (1952) Folder 28: S (1951-1952) Folder 29: T, U, V (1951-1952) Folder 30: W (1952) Folder 31: Misc. (1954-1955) Folder 32: A (1955) Folder 33: B (1955) Folder 34: C (1954-1955) Folder 35: D (1955) Folder 36: E, F (1955) Folder 37: H, I, J (1955-1956) Folder 38: K, L (1955) Folder 39: M (1955) D-299 Copyright ©2014 Regents of the University of California 2 Folder 40: N, O (1955) Folder 41: P, Q (1954-1955) Folder 42: R (1955) Folder 43: S (1955) Folder 44: T, U, V (1955) Folder 45: W (1955) Folder 46: Y, Z (1955?) Folder 47: Misc. -

Igor Gonçalves Lima1, Natanael Costa Rebouças1, Rayane De Tasso Moreira Ribeiro2,3 & Maria Iracema Bezerra Loiola1,4
Rodriguésia 71: e01782018. 2020 http://rodriguesia.jbrj.gov.br DOI: http://dx.doi.org/10.1590/2175-7860202071007 Artigo Original / Original Paper Flora do Ceará, Brasil: Phyllanthaceae Flora of Ceará, Brazil: Phyllanthaceae Igor Gonçalves Lima1, Natanael Costa Rebouças1, Rayane de Tasso Moreira Ribeiro2,3 & Maria Iracema Bezerra Loiola1,4 Resumo Apresentamos o levantamento florístico de Phyllanthaceae no estado do Ceará, como parte do projeto Flora do Ceará: conhecer para conservar. O estudo baseou-se na análise de coleções depositadas em herbários e observação de populações naturais no campo. Phyllanthaceae está representada por 14 espécies e quatro gêneros: Hieronyma (2), Margaritaria (1), Phyllanthus (10) e Savia (1). A ocorrência das espécies Hieronyma alchorneoides, H. oblonga e Margaritaria nobilis, assim como Phyllanthus acuminatus, P. carmenluciae, P. caroliniensis, P. heteradenius e P. stipulatus constituem novos registros para o estado. As espécies ocorrem preferencialmente em floresta ombrófila densa (mata úmida) e savana estépica (caatinga), havendo registros em Unidades de Conservação do Ceará. Palavras-chave: Caatinga, diversidade, Hieronyma, Malpighiales, Margaritaria, nordeste do Brasil, Phyllanthus, Savia. Abstract We present the floristic survey of Phyllanthaceae in the state of Ceará, as part of the project Flora of Ceará: knowledge towards conservation. The study was based on the analysis of collections deposited in herbarium collections and observation of natural populations in the field. Phyllanthaceae is represented by 14 species and four genera: Hieronyma (2), Margaritaria (1), Phyllanthus (10) and Savia (1). The ocurrence of Hieronyma alchorneoides, H. oblonga and Margaritaria nobilis, as well as Phyllanthus acuminatus, P. carmenluciae, P. caroliniensis, P. heteradenius and P. stipulatus are new records for the state. -

Las Euphorbiaceae De Colombia Biota Colombiana, Vol
Biota Colombiana ISSN: 0124-5376 [email protected] Instituto de Investigación de Recursos Biológicos "Alexander von Humboldt" Colombia Murillo A., José Las Euphorbiaceae de Colombia Biota Colombiana, vol. 5, núm. 2, diciembre, 2004, pp. 183-199 Instituto de Investigación de Recursos Biológicos "Alexander von Humboldt" Bogotá, Colombia Disponible en: http://www.redalyc.org/articulo.oa?id=49150203 Cómo citar el artículo Número completo Sistema de Información Científica Más información del artículo Red de Revistas Científicas de América Latina, el Caribe, España y Portugal Página de la revista en redalyc.org Proyecto académico sin fines de lucro, desarrollado bajo la iniciativa de acceso abierto Biota Colombiana 5 (2) 183 - 200, 2004 Las Euphorbiaceae de Colombia José Murillo-A. Instituto de Ciencias Naturales, Universidad Nacional de Colombia, Apartado 7495, Bogotá, D.C., Colombia. [email protected] Palabras Clave: Euphorbiaceae, Phyllanthaceae, Picrodendraceae, Putranjivaceae, Colombia Euphorbiaceae es una familia muy variable El conocimiento de la familia en Colombia es escaso, morfológicamente, comprende árboles, arbustos, lianas y para el país sólo se han revisado los géneros Acalypha hierbas; muchas de sus especies son componentes del bos- (Cardiel 1995), Alchornea (Rentería 1994) y Conceveiba que poco perturbado, pero también las hay de zonas alta- (Murillo 1996). Por otra parte, se tiene el catálogo de las mente intervenidas y sólo Phyllanthus fluitans es acuáti- especies de Croton (Murillo 1999) y la revisión de la ca. -

Sexual Systems of Plants in a Brazilian Montane Forest
Floresta e Ambiente 2019; 26(Spec No 1): e20180394 https://doi.org/10.1590/2179-8087.039418 ISSN 2179-8087 (online) Original Article Conservation of Nature Sexual Systems of Plants in a Brazilian Montane Forest Monique Perini1 , Henrique Machado Dias2 , Sustanis Horn Kunz2 1Programa de Pós-graduação em Biologia Vegetal, Instituto de Ciências Biológicas, Universidade Federal de Minas Gerais – UFMG, Belo Horizonte/MG, Brasil 2Departamento de Ciências Florestais e da Madeira, Universidade Federal do Espírito Santo – UFES, Jerônimo Monteiro/ES, Brasil ABSTRACT In this study, vegetation reproduction has been investigated in order to understand aspects of speciation, structuring and composition of plant communities. Thus, we sought to characterize the frequency of sexual systems from species recorded in seed rain occurring in a tropical rainforest (Atlantic Forest) in Caparaó National Park, Espírito Santo State, Brazil. We collected the seed rain for twelve months, classified and recorded the species for: sexual system; pollination and dispersion syndrome; and fruit type. Then we measured the correlation between these attributes through correspondence analysis. Regarding sexual systems, 71% were hermaphrodites, 13% dioecious, and 11% monoecious. Hermaphrodites are best associated with pollination, dispersion and fruit types, represented by 65% of data variance. This study may contribute to elaborating management and conservation programs taking into account the interaction of plants with the local fauna. Keywords: seed rain, reproductive ecology, woody layer, Caparaó National Park, Atlantic Forest. Creative Commons License. All the contents of this journal, except where otherwise noted, is licensed under a Creative Commons Attribution License. 2/10 Perini M, Dias HM, Kunz SH Floresta e Ambiente 2019; 26(Spec No 1): e20180394 1. -

Euphorbiaceae, in Subfamily Phyllanthoideae
Botanical Journal of the Linnean Society (1987), 94: 111-126. With 8 figures Wood anatomy of the Euphorbiaceae, in particular of the subfamily Phyllanthoideae Alberta+M.W. Mennega Institute of Systematic Botany, Rijksuniversiteit, Utrecht, The Netherlands Received June 1986, accepted for Publication September 1986 M. 1987. Wood of the in MENNEGA, ALBERTA, W., anatomy Euphorbiaceae, particular of the The in of subfamily Phyllanthoideae. great variety wood structure the large family Euphorbiaceae makes it impossible to describe briefly a generalwood pattern. Nevertheless, a more or less clear division into four anatomical groups can be made. A short overview is given of the wood structure of the uni-ovulate subfamilies Acalyphoideae, Crotonoideae, and Euphorbioideae, following the classification by Webster. These subfamilies be their cannot distinguished by anatomy. The paper is mainly devoted to the bi-ovulate subfamily Within this two be on the basis of their wood Phyllanthoideae. subfamily, groups can recognized the anatomy: Aporusa type with a great number of primitive characters, and the Glochidion type, in which features such scalariform vessel short primitive as perforation plates are absent. A description of the 13 tribes is well for of the succession of the tribes. In given as as suggestions rearrangement tribes several some taxa are anomalous and, on anatomical evidence, exclusion of some genera, sometimes with assignment to another tribe, is suggested. Fibre Additional key words: types parenchyma Stilaginaceae Uapacaceae Contents Introduction 112 Material 112 Characters used for establishing an evolutionary grouping of the tribes in the subfamily Phyllanthoideae 112 Short descriptions of the tribes of Phyllanthoideae 116 Tribe 8. Aporuseae (Lindl. -

Unraveling the Biogeographical History of Chrysobalanaceae from Plastid Genomes1
RESEARCH ARTICLE AMERICAN JOURNAL OF BOTANY Unraveling the biogeographical history of Chrysobalanaceae from plastid genomes1 Léa Bardon 2 , Cynthia Sothers 3 , Ghillean T. Prance 3 , Pierre-Jean G. Malé 4 , Zhenxiang Xi 5 , Charles C. Davis 5 , Jerome Murienne 2 , Roosevelt García-Villacorta 6 , Eric Coissac 7 , Sébastien Lavergne 7 , and Jérôme Chave 2,8 PREMISE OF THE STUDY: The complex geological and climatic history of the Neotropics has had major implications on the diversifi cation of plant lineages. Chrysobalanaceae is a pantropical family of trees and shrubs with 75% of its 531 species found in the Neotropics, and a time-calibrated phylogeny of this family should shed light on the tempo of diversifi cation in the Neotropical fl ora. Previously published phylogenetic hypotheses of this family were poorly supported, and its biogeography remains unclear. METHODS: We assembled the complete plastid genome of 51 Chrysobalanaceae species, and increased taxon sampling by Sanger-sequencing of fi ve plastid regions for an additional 88 species. We generated a time-calibrated tree including all 139 Chrsyobalanaceae species and 23 outgroups. We then conducted an ancestral area reconstruction analysis and estimated diversifi cation rates in the family. KEY RESULTS: The tree generated with the plastid genome alignment was almost fully resolved. It supports the polyphyly of Licania and Hirtella . The family has diversifi ed starting around the Eocene-Oligocene transition. An ancestral area reconstruction confi rms a Paleotropical origin for Chrysobalanaceae with several transoceanic dispersal events. The main Neotropical clade likely resulted from a single migration event from Africa around 28 mya ago, which subsequently underwent rapid diversifi cation. -
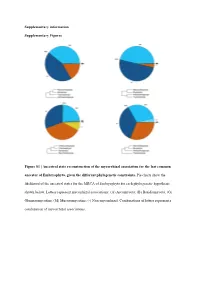
Ancestral State Reconstruction of the Mycorrhizal Association for the Last Common Ancestor of Embryophyta, Given the Different Phylogenetic Constraints
Supplementary information Supplementary Figures Figure S1 | Ancestral state reconstruction of the mycorrhizal association for the last common ancestor of Embryophyta, given the different phylogenetic constraints. Pie charts show the likelihood of the ancestral states for the MRCA of Embryophyta for each phylogenetic hypothesis shown below. Letters represent mycorrhizal associations: (A) Ascomycota; (B) Basidiomycota; (G) Glomeromycotina; (M) Mucoromycotina; (-) Non-mycorrhizal. Combinations of letters represent a combination of mycorrhizal associations. Austrocedrus chilensis Chamaecyparis obtusa Sequoiadendron giganteum Prumnopitys taxifolia Prumnopitys Prumnopitys montana Prumnopitys Prumnopitys ferruginea Prumnopitys Araucaria angustifolia Araucaria Dacrycarpus dacrydioides Dacrycarpus Taxus baccata Podocarpus oleifolius Podocarpus Afrocarpus falcatus Afrocarpus Ephedra fragilis Nymphaea alba Nymphaea Gnetum gnemon Abies alba Abies balsamea Austrobaileya scandens Austrobaileya Abies nordmanniana Thalictrum minus Thalictrum Abies homolepis Caltha palustris Caltha Abies magnifica ia repens Ranunculus Abies religiosa Ranunculus montanus Ranunculus Clematis vitalba Clematis Keteleeria davidiana Anemone patens Anemone Tsuga canadensis Vitis vinifera Vitis Tsuga mertensiana Saxifraga oppositifolia Saxifraga Larix decidua Hypericum maculatum Hypericum Larix gmelinii Phyllanthus calycinus Phyllanthus Larix kaempferi Hieronyma oblonga Hieronyma Pseudotsuga menziesii Salix reinii Salix Picea abies Salix polaris Salix Picea crassifolia Salix herbacea