Horizontal and Elevational Phylogeographic Patterns of Himalayan and Southeast Asian Forest Passerines (Aves: Passeriformes)
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Printable PDF Format
Field Guides Tour Report Taiwan 2020 Feb 1, 2020 to Feb 12, 2020 Phil Gregory & local guide Arco Huang For our tour description, itinerary, past triplists, dates, fees, and more, please VISIT OUR TOUR PAGE. This gorgeous male Swinhoe's Pheasant was one of the birds of the trip! We found a pair of these lovely endemic pheasants at Dasyueshan. Photo by guide Phil Gregory. This was a first run for the newly reactivated Taiwan tour (which we last ran in 2006), with a new local organizer who proved very good and enthusiastic, and knew the best local sites to visit. The weather was remarkably kind to us and we had no significant daytime rain, somewhat to my surprise, whilst temperatures were pretty reasonable even in the mountains- though it was cold at night at Dasyueshan where the unheated hotel was a bit of a shock, but in a great birding spot, so overall it was bearable. Fog on the heights of Hohuanshan was a shame but at least the mid and lower levels stayed clear. Otherwise the lowland sites were all good despite it being very windy at Hengchun in the far south. Arco and I decided to use a varied assortment of local eating places with primarily local menus, and much to my amazement I found myself enjoying noodle dishes. The food was a highlight in fact, as it was varied, often delicious and best of all served quickly whilst being both hot and fresh. A nice adjunct to the trip, and avoided losing lots of time with elaborate meals. -

ED45E Rare and Scarce Species Hierarchy.Pdf
104 Species 55 Mollusc 8 Mollusc 334 Species 181 Mollusc 28 Mollusc 44 Species 23 Vascular Plant 14 Flowering Plant 45 Species 23 Vascular Plant 14 Flowering Plant 269 Species 149 Vascular Plant 84 Flowering Plant 13 Species 7 Mollusc 1 Mollusc 42 Species 21 Mollusc 2 Mollusc 43 Species 22 Mollusc 3 Mollusc 59 Species 30 Mollusc 4 Mollusc 59 Species 31 Mollusc 5 Mollusc 68 Species 36 Mollusc 6 Mollusc 81 Species 43 Mollusc 7 Mollusc 105 Species 56 Mollusc 9 Mollusc 117 Species 63 Mollusc 10 Mollusc 118 Species 64 Mollusc 11 Mollusc 119 Species 65 Mollusc 12 Mollusc 124 Species 68 Mollusc 13 Mollusc 125 Species 69 Mollusc 14 Mollusc 145 Species 81 Mollusc 15 Mollusc 150 Species 84 Mollusc 16 Mollusc 151 Species 85 Mollusc 17 Mollusc 152 Species 86 Mollusc 18 Mollusc 158 Species 90 Mollusc 19 Mollusc 184 Species 105 Mollusc 20 Mollusc 185 Species 106 Mollusc 21 Mollusc 186 Species 107 Mollusc 22 Mollusc 191 Species 110 Mollusc 23 Mollusc 245 Species 136 Mollusc 24 Mollusc 267 Species 148 Mollusc 25 Mollusc 270 Species 150 Mollusc 26 Mollusc 333 Species 180 Mollusc 27 Mollusc 347 Species 189 Mollusc 29 Mollusc 349 Species 191 Mollusc 30 Mollusc 365 Species 196 Mollusc 31 Mollusc 376 Species 203 Mollusc 32 Mollusc 377 Species 204 Mollusc 33 Mollusc 378 Species 205 Mollusc 34 Mollusc 379 Species 206 Mollusc 35 Mollusc 404 Species 221 Mollusc 36 Mollusc 414 Species 228 Mollusc 37 Mollusc 415 Species 229 Mollusc 38 Mollusc 416 Species 230 Mollusc 39 Mollusc 417 Species 231 Mollusc 40 Mollusc 418 Species 232 Mollusc 41 Mollusc 419 Species 233 -

Biodiversity Climate Change Impacts Report Card Technical Paper 12. the Impact of Climate Change on Biological Phenology In
Sparks Pheno logy Biodiversity Report Card paper 12 2015 Biodiversity Climate Change impacts report card technical paper 12. The impact of climate change on biological phenology in the UK Tim Sparks1 & Humphrey Crick2 1 Faculty of Engineering and Computing, Coventry University, Priory Street, Coventry, CV1 5FB 2 Natural England, Eastbrook, Shaftesbury Road, Cambridge, CB2 8DR Email: [email protected]; [email protected] 1 Sparks Pheno logy Biodiversity Report Card paper 12 2015 Executive summary Phenology can be described as the study of the timing of recurring natural events. The UK has a long history of phenological recording, particularly of first and last dates, but systematic national recording schemes are able to provide information on the distributions of events. The majority of data concern spring phenology, autumn phenology is relatively under-recorded. The UK is not usually water-limited in spring and therefore the major driver of the timing of life cycles (phenology) in the UK is temperature [H]. Phenological responses to temperature vary between species [H] but climate change remains the major driver of changed phenology [M]. For some species, other factors may also be important, such as soil biota, nutrients and daylength [M]. Wherever data is collected the majority of evidence suggests that spring events have advanced [H]. Thus, data show advances in the timing of bird spring migration [H], short distance migrants responding more than long-distance migrants [H], of egg laying in birds [H], in the flowering and leafing of plants[H] (although annual species may be more responsive than perennial species [L]), in the emergence dates of various invertebrates (butterflies [H], moths [M], aphids [H], dragonflies [M], hoverflies [L], carabid beetles [M]), in the migration [M] and breeding [M] of amphibians, in the fruiting of spring fungi [M], in freshwater fish migration [L] and spawning [L], in freshwater plankton [M], in the breeding activity among ruminant mammals [L] and the questing behaviour of ticks [L]. -

Disaggregation of Bird Families Listed on Cms Appendix Ii
Convention on the Conservation of Migratory Species of Wild Animals 2nd Meeting of the Sessional Committee of the CMS Scientific Council (ScC-SC2) Bonn, Germany, 10 – 14 July 2017 UNEP/CMS/ScC-SC2/Inf.3 DISAGGREGATION OF BIRD FAMILIES LISTED ON CMS APPENDIX II (Prepared by the Appointed Councillors for Birds) Summary: The first meeting of the Sessional Committee of the Scientific Council identified the adoption of a new standard reference for avian taxonomy as an opportunity to disaggregate the higher-level taxa listed on Appendix II and to identify those that are considered to be migratory species and that have an unfavourable conservation status. The current paper presents an initial analysis of the higher-level disaggregation using the Handbook of the Birds of the World/BirdLife International Illustrated Checklist of the Birds of the World Volumes 1 and 2 taxonomy, and identifies the challenges in completing the analysis to identify all of the migratory species and the corresponding Range States. The document has been prepared by the COP Appointed Scientific Councilors for Birds. This is a supplementary paper to COP document UNEP/CMS/COP12/Doc.25.3 on Taxonomy and Nomenclature UNEP/CMS/ScC-Sc2/Inf.3 DISAGGREGATION OF BIRD FAMILIES LISTED ON CMS APPENDIX II 1. Through Resolution 11.19, the Conference of Parties adopted as the standard reference for bird taxonomy and nomenclature for Non-Passerine species the Handbook of the Birds of the World/BirdLife International Illustrated Checklist of the Birds of the World, Volume 1: Non-Passerines, by Josep del Hoyo and Nigel J. Collar (2014); 2. -

Complete Mitochondrial Genome of the Chinese Hwamei Garrulax Canorus
Complete mitochondrial genome of the Chinese Hwamei Garrulax canorus (Aves: Passeriformes): the first representative of the Leiothrichidae family with a duplicated control region D.S. Chen1*, C.J. Qian1*, Q.Q. Ren1,2, P. Wang1, J. Yuan1, L. Jiang1, D. Bi1, Q. Zhang1, Y. Wang1 and X.Z. Kan1,2 1Provincial Key Laboratory of the Conservation and Exploitation Research of Biological Resources in Anhui, College of Life Sciences, Anhui Normal University, Wuhu, China 2The Institute of Bioinformatics, College of Life Sciences, Anhui Normal University, Wuhu, China *These authors contributed equally to this study. Corresponding author: X.Z. Kan E-mail: [email protected] Genet. Mol. Res. 14 (3): 8964-8976 (2015) Received January 11, 2015 Accepted May 8, 2015 Published August 7, 2015 DOI http://dx.doi.org/10.4238/2015.August.7.5 ABSTRACT. The Chinese Hwamei Garrulax canorus, a member of the family Leiothrichidae, is commonly found in central and southern China, northern Indochina, and on Hainan Island. In this study, we sequenced the complete mitochondrial genome of G. canorus. The circular mitochondrial genome is 17,785 bp in length and includes 13 protein-coding genes, 22 transfer RNA (tRNA) genes, and two ribosomal RNA genes. In addition, two copies of highly Genetics and Molecular Research 14 (3): 8964-8976 (2015) ©FUNPEC-RP www.funpecrp.com.br Mitogenome of Garrulax canorus 8965 similar putative control regions were observed in the mitochondrial genome. As found in other vertebrates, most of the genes are coded on the H-strand, except for one protein-coding gene (nad6; NADH dehydrogenase subunit 6) and eight tRNA genes (tRNAGln, tRNAAla, tRNAAsn, tRNACys, tRNATyr, tRNASer(UCN), tRNAPro, and tRNAGlu). -

Thailand Highlights 14Th to 26Th November 2019 (13 Days)
Thailand Highlights 14th to 26th November 2019 (13 days) Trip Report Siamese Fireback by Forrest Rowland Trip report compiled by Tour Leader: Forrest Rowland Trip Report – RBL Thailand - Highlights 2019 2 Tour Summary Thailand has been known as a top tourist destination for quite some time. Foreigners and Ex-pats flock there for the beautiful scenery, great infrastructure, and delicious cuisine among other cultural aspects. For birders, it has recently caught up to big names like Borneo and Malaysia, in terms of respect for the avian delights it holds for visitors. Our twelve-day Highlights Tour to Thailand set out to sample a bit of the best of every major habitat type in the country, with a slight focus on the lush montane forests that hold most of the country’s specialty bird species. The tour began in Bangkok, a bustling metropolis of winding narrow roads, flyovers, towering apartment buildings, and seemingly endless people. Despite the density and throng of humanity, many of the participants on the tour were able to enjoy a Crested Goshawk flight by Forrest Rowland lovely day’s visit to the Grand Palace and historic center of Bangkok, including a fun boat ride passing by several temples. A few early arrivals also had time to bird some of the urban park settings, even picking up a species or two we did not see on the Main Tour. For most, the tour began in earnest on November 15th, with our day tour of the salt pans, mudflats, wetlands, and mangroves of the famed Pak Thale Shore bird Project, and Laem Phak Bia mangroves. -

Resolving Phylogenetic Relationships Within Passeriformes Based on Mitochondrial Genes and Inferring the Evolution of Their Mitogenomes in Terms of Duplications
GBE Resolving Phylogenetic Relationships within Passeriformes Based on Mitochondrial Genes and Inferring the Evolution of Their Mitogenomes in Terms of Duplications Paweł Mackiewicz1,*, Adam Dawid Urantowka 2, Aleksandra Kroczak1,2, and Dorota Mackiewicz1 1Department of Bioinformatics and Genomics, Faculty of Biotechnology, University of Wrocław, Poland 2Department of Genetics, Wroclaw University of Environmental and Life Sciences, Poland *Corresponding author: E-mail: pamac@smorfland.uni.wroc.pl. Accepted: September 30, 2019 Abstract Mitochondrial genes are placed on one molecule, which implies that they should carry consistent phylogenetic information. Following this advantage, we present a well-supported phylogeny based on mitochondrial genomes from almost 300 representa- tives of Passeriformes, the most numerous and differentiated Aves order. The analyses resolved the phylogenetic position of para- phyletic Basal and Transitional Oscines. Passerida occurred divided into two groups, one containing Paroidea and Sylvioidea, whereas the other, Passeroidea and Muscicapoidea. Analyses of mitogenomes showed four types of rearrangements including a duplicated control region (CR) with adjacent genes. Mapping the presence and absence of duplications onto the phylogenetic tree revealed that the duplication was the ancestral state for passerines and was maintained in early diverged lineages. Next, the duplication could be lost and occurred independently at least four times according to the most parsimonious scenario. In some lineages, two CR copies have been inherited from an ancient duplication and highly diverged, whereas in others, the second copy became similar to the first one due to concerted evolution. The second CR copies accumulated over twice as many substitutions as the first ones. However, the second CRs were not completely eliminated and were retained for a long time, which suggests that both regions can fulfill an important role in mitogenomes. -

Ecological Assessments in the B+WISER Sites
Ecological Assessments in the B+WISER Sites (Northern Sierra Madre Natural Park, Upper Marikina-Kaliwa Forest Reserve, Bago River Watershed and Forest Reserve, Naujan Lake National Park and Subwatersheds, Mt. Kitanglad Range Natural Park and Mt. Apo Natural Park) Philippines Biodiversity & Watersheds Improved for Stronger Economy & Ecosystem Resilience (B+WISER) 23 March 2015 This publication was produced for review by the United States Agency for International Development. It was prepared by Chemonics International Inc. The Biodiversity and Watersheds Improved for Stronger Economy and Ecosystem Resilience Program is funded by the USAID, Contract No. AID-492-C-13-00002 and implemented by Chemonics International in association with: Fauna and Flora International (FFI) Haribon Foundation World Agroforestry Center (ICRAF) The author’s views expressed in this publication do not necessarily reflect the views of the United States Agency for International Development or the United States Government. Ecological Assessments in the B+WISER Sites Philippines Biodiversity and Watersheds Improved for Stronger Economy and Ecosystem Resilience (B+WISER) Program Implemented with: Department of Environment and Natural Resources Other National Government Agencies Local Government Units and Agencies Supported by: United States Agency for International Development Contract No.: AID-492-C-13-00002 Managed by: Chemonics International Inc. in partnership with Fauna and Flora International (FFI) Haribon Foundation World Agroforestry Center (ICRAF) 23 March -
![Use of Field Recorded Sounds in the Assessment of Forest Birds in Palawan, Philippines ______Required in the Field [19]](https://docslib.b-cdn.net/cover/0725/use-of-field-recorded-sounds-in-the-assessment-of-forest-birds-in-palawan-philippines-required-in-the-field-19-570725.webp)
Use of Field Recorded Sounds in the Assessment of Forest Birds in Palawan, Philippines ______Required in the Field [19]
Asia Pacific Journal of Multidisciplinary Research, Vol. 7, No. 2, May, 2019 _____________________________________________________________________________________________________________________ Asia Pacific Journal of Use of Field Recorded Sounds in the Multidisciplinary Research Assessment of Forest Birds in Palawan, Vol. 7 No.2, 24-31 May 2019 Philippines P-ISSN 2350-7756 E-ISSN 2350-8442 Alejandro A. Bernardo Jr. www.apjmr.com College of Arts and Sciences, Western Philippines University, CHED Recognized Journal Aborlan, Palawan, Philippines ASEAN Citation Index [email protected] Date Received: August 3, 2018; Date Revised: February 8, 2019 Abstract -The uses of bioacoustics in biological applications are getting popular in research communities. Among such application is the use of sound recordings in avifaunal researches. This research explored the possibility of using the sound recording in the assessment of forest birds in Palawan by comparing it in widely used Point Count Method (PCM). To compare the two methods, a simultaneous point count and sound recording surveys from February to November 2017 in the forested slopes of Victoria-Anipahan Mountain in Aborlan, Palawan were conducted. The Sound Recording Method (SRM) listed slightly lower species richness than the PCM, but the difference in the mean number of species was not significant (F1,49=1.05, p > 0.05). The SRM was found to be biased towards noisy and loud calling bird species but it failed to detect the silent and rarely calling species. SRM was also equally sensitive as compared to PCM in detecting endemic and high conservation priority species. Because of these, it was recognized that SRM could be used as one of the alternative methods in forest bird assessment particularly if the concern is avifaunal species richness. -
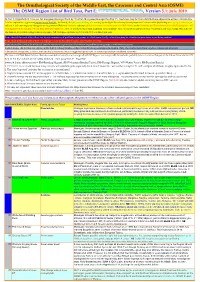
ORL 5.1 Hypothetical Spp Final Draft01a.Xlsx
The Ornithological Society of the Middle East, the Caucasus and Central Asia (OSME) The OSME Region List of Bird Taxa, Part E: , Version 5.1: July 2019 In Part E, Hypothetical Taxa, we list non-passerines (prefixed by 'N') first, then passerines (prefixed by 'P'). Such taxa may be from distributions adjacent to or have extended to A fuller explanation is given in Explanation of the ORL, but briefly, Bright green shading of a row (eg Syrian Ostrich) indicates former presence of a taxon in the OSME Region. Light gold shading in column A indicates sequence change from the previous ORL issue. Red font indicates added information since the previous ORL version or the Conservation Threat Status (Critically Endangered = CE, Endangered = E, Vulnerable = V and Data Deficient = DD only). Not all synonyms have been examined. Serial numbers (SN) are merely an administrative convenience and may change. Please do not cite them in any formal correspondence or papers. NB: Compass cardinals (eg N = north, SE = southeast) are used. Rows shaded thus and with yellow text denote summaries of problem taxon groups in which some closely-related taxa may be of indeterminate status or are being studied. Rows shaded thus and with yellow text indicate recent or data-driven major conservation concerns. Rows shaded thus and with white text contain additional explanatory information on problem taxon groups as and when necessary. English names shaded thus are species on BirdLife Tracking Database, http://seabirdtracking.org/mapper/index.php. Only a few individuals from very few colonies are involved. A broad dark orange line, as below, indicates the last taxon in a new or suggested species split, or where sspp are best considered separately. -

Assessment and Conservation of Threatened Bird Species at Laojunshan, Sichuan, China
CLP Report Assessment and conservation of threatened bird species at Laojunshan, Sichuan, China Submitted by Jie Wang Institute of Zoology, Chinese Academy of Sciences, Beijing, P.R.China E-mail:[email protected] To Conservation Leadership Programme, UK Contents 1. Summary 2. Study area 3. Avian fauna and conservation status of threatened bird species 4. Habitat analysis 5. Ecological assessment and community education 6. Outputs 7. Main references 8. Acknowledgements 1. Summary Laojunshan Nature Reserve is located at Yibin city, Sichuan province, south China. It belongs to eastern part of Liangshan mountains and is among the twenty-five hotspots of global biodiversity conservation. The local virgin alpine subtropical deciduous forests are abundant, which are actually rare at the same latitudes and harbor a tremendous diversity of plant and animal species. It is listed as a Global 200 ecoregion (WWF), an Important Bird Area (No. CN205), and an Endemic Bird Area (No. D14) (Stattersfield, et al . 1998). However, as a nature reserve newly built in 1999, it is only county-level and has no financial support from the central government. Especially, it is quite lack of scientific research, for example, the avifauna still remains unexplored except for some observations from bird watchers. Furthermore, the local community is extremely poor and facing modern development pressures, unmanaged human activities might seriously disturb the local ecosystem. We conducted our project from April to June 2007, funded by Conservation Leadership Programme. Two fieldwork strategies were used: “En bloc-Assessment” to produce an avifauna census and ecological assessments; "Special Survey" to assess the conservation status of some threatened endemic bird species. -

SICHUAN (Including Northern Yunnan)
Temminck’s Tragopan (all photos by Dave Farrow unless indicated otherwise) SICHUAN (Including Northern Yunnan) 16/19 MAY – 7 JUNE 2018 LEADER: DAVE FARROW The Birdquest tour to Sichuan this year was a great success, with a slightly altered itinerary to usual due to the closure of Jiuzhaigou, and we enjoyed a very smooth and enjoyable trip around the spectacular and endemic-rich mountain and plateau landscapes of this striking province. Gamebirds featured strongly with 14 species seen, the highlights of them including a male Temminck’s Tragopan grazing in the gloom, Chinese Monal trotting across high pastures, White Eared and Blue Eared Pheasants, Lady Amherst’s and Golden Pheasants, Chinese Grouse and Tibetan Partridge. Next were the Parrotbills, with Three-toed, Great and Golden, Grey-hooded and Fulvous charming us, Laughingthrushes included Red-winged, Buffy, Barred, Snowy-cheeked and Plain, we saw more Leaf Warblers than we knew what to do with, and marvelled at the gorgeous colours of Sharpe’s, Pink-rumped, Vinaceous, Three-banded and Red-fronted Rosefinches, the exciting Przevalski’s Finch, the red pulse of Firethroats plus the unreal blue of Grandala. Our bird of the trip? Well, there was that Red Panda that we watched for ages! 1 BirdQuest Tour Report: Sichuan Including Northern Yunnan 2018 www.birdquest-tours.com Our tour began with a short extension in Yunnan, based in Lijiang city, with the purpose of finding some of the local specialities including the rare White-speckled Laughingthrush, which survives here in small numbers. Once our small group had arrived in the bustling city of Lijiang we began our birding in an area of hills that had clearly been totally cleared of forest in the fairly recent past, with a few trees standing above the hillsides of scrub.