Arecaceae- Arecoideae- Attaleinae), Amazon
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Musky Rat-Kangaroos, Hypsiprymnodon Moschatus: Cursorial Frugivores in Australia's Wet-Tropical Rain Forests
ResearchOnline@JCU This file is part of the following reference: Dennis, Andrew James (1997) Musky Rat-kangaroos, hypsiprymnodon moschatus: cursorial frugivores in Australia's wet-tropical rain forests. PhD thesis, James Cook University. Access to this file is available from: http://eprints.jcu.edu.au/17401/ If you believe that this work constitutes a copyright infringement, please contact [email protected] and quote http://eprints.jcu.edu.au/17401/ Chapter 11 MUSKY RAT-KANGAROOS: CURSORIAL FRUGIVORES How do Musky Rat-kangaroos Relate !Q..their Environment ? Musky Rat-kangaroos can be classified as frugivo res because fr ui ts and seeds accounted fo r the bul k of their diet th roughout the year (Chapter 3). In addi tion, they scatterhoarded many fruits and seeds, to the benefit of at least some species of plants (Chapters 8 & 9). They consumed most of the available frui ts which had a fleshy pericarp or ani but also included the seeds of some species that did not. They ate fruits from over half the species producing fruits on my study site, many of those they did not eat were wind dispersed, housed in hard, indehiscent pods or had furry. dehiscent pods. In addition, some fleshy drupes were not consumed. Like many other frugivores, Musky Rat-kangaroos supplemented their diet from other sources, particularly when the seasonal availabil ity of fruits is at its min imum (see Terborgh 1983). During the late wet, co ld an d early dry seasons, when frui t abundance was mini mal, their search effort was random with respect to fr ui t fall s (Figure 3.8; Table 3.2). -

Pelagodoxa Henryana (Arecaceae): a Supplement of Additional Photographs and Figures to the 2019 Article in the Journal PALMS
PALMARBOR Hodel et al.: Pelagodoxa supplement 2019-1: 1-24 Pelagodoxa henryana (Arecaceae): A Supplement of Additional Photographs and Figures to the 2019 Article in the Journal PALMS DONALD R. HODEL, JEAN-FRANCOIS BUTAUD, CRAIG E. BARRETT, MICHAEL H. GRAYUM, JAMES KOMEN, DAVID H. LORENCE, JEFF MARCUS, AND ARIITEUIRA FALCHETTO With its large, initially undivided leaves; big, curious, warty fruits; monotypic nature; and mysterious, remote, island habitat, Pelagodoxa henryana has long fascinated palm botanists, collectors and growers, and been one of the holy grails of all who have an interest in palms. The possibility of a second species of Pelagodoxa has generated a substantial amount of interest but the recent literature on the subject has dismissed this prospect and accepted or recognized only one species. However, for 40 years the senior author has propagated and grown P. henryana nearly side by side with a second species of the genus, first in Hawaii, U.S.A and later at his wife’s home in Papeari, Tahiti, French Polynesia, allowing ample opportunity to compare and contrast the two species at various stages of development. An article we wrote reassessing the genus Pelagodoxa was published in the journal PALMS [Hodel et al., Reassessment of Pelagodoxa, PALMS 63(3): 113-146. 2019]. In it we document substantial and critical differences between the two species, P. henryana and P. mesocarpa, establish the validity and resurrect the name of the second species from synonymy, discuss molecular data, phylogeny and phytogeography, ethnobotany and conservation of Pelagodoxa and what impact, if any, they might have had in its speciation and insular distribution. -
![Syagrus Romanzoffiana [Cham.] Glassman](https://docslib.b-cdn.net/cover/5715/syagrus-romanzoffiana-cham-glassman-115715.webp)
Syagrus Romanzoffiana [Cham.] Glassman
SCIENTIFIC note Doi: https://doi.org/10.17584/rcch.2019v13i3.8363 Pre-depulping and depulping treatments and the emergence of queen palm seeds (Syagrus romanzoffiana [Cham.] Glassman) Tratamiento de pre-despulpado y despulpado sobre la emergencia de semillas de palma reina (Syagrus romanzoffiana [Cham.] Glassman) LUCAS MARQUEZAN NASCIMENTO1 EDUARDO PRADI VENDRUSCOLO2, 4 LUIZ FERNANDES CARDOSO CAMPOS1 LISMAÍRA GONÇALVES CAIXETA GARCIA1 LARISSA LEANDRO PIRES1 ALEXANDER SELEGUINI3 Syagrus romanzoffiana under conditions of Brazilian Cerrado. Photo: L.M. Nascimento ABSTRACT The propagation of the palm Syagrus romanzoffiano is done sexually with seeds, making the process of obtai- ning new plants slow and difficult, especially on large scales. In addition, seed germination is slow, uneven and susceptible to degradation and loss of vigor because of embryo deterioration, even under laboratory conditions. As a result of the lack of information on efficient depulping methods for queen palm fruits, the present study aimed to establish a depulping methodology that is less aggressive to embryos, maintaining emergence quality. This experiment was carried out in Goiânia, Brazil, using fruits from eight stock plants submitted to three pre-depulping treatments (control, fermentation and drying) and two depulping me- thods (industrial depulping and concrete-mixer with the addition of gravel). After the different pre-sowing processes, the fresh and dry pyrenes mass, remaining fibers adhered to the pyrene and seedling emergence were evaluated. The pulper removed an average of 45% more pyrene pulp than the concrete mixer. However, these methodologies did not result in differences in the emergence of plants, which was affected only by the pre-depulping treatment, with superiority in the use of fresh fruits. -
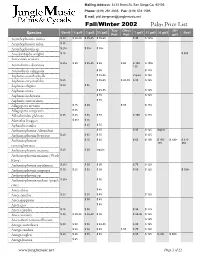
Winter-Fall Sale 2002 Palm Trees-Web
Mailing Address: 3233 Brant St. San Diego Ca, 92103 Phone: (619) 291 4605 Fax: (619) 574 1595 E mail: [email protected] Fall/Winter 2002 Palm Price List Tree Citrus 25/+ Band$ 1 gal$ 2 gal$ 3/5 gal$ 7 gal$ 15 gal$ 20 gal$ Box$ Species Pot$ Pot$ gal$ Acanthophoenix crinita $ 30 $ 30-40 $ 35-45 $ 55-65 $ 95 $ 125+ Acanthophoenix rubra $ 35 Acanthophoenix sp. $ 25+ $ 35+ $ 55+ Acoelorrhaphe wrightii $ 15 $ 300 Acrocomia aculeata $ 25+ $ 35 $ 35-45 $ 65 $ 65 $ 100- $ 150+ Actinokentia divaricata 135 Actinorhytis calapparia $ 55 $ 125 Aiphanes acanthophylla $ 45-55 inquire $ 125 Aiphanes caryotaefolia $ 25 $ 55-65 $ 45-55 $ 85 $ 125 Aiphanes elegans $ 20 $ 35 Aiphanes erosa $ 45-55 $ 125 Aiphanes lindeniana $ 55 $ 125 Aiphanes vincentsiana $ 55 Allagoptera arenaria $ 25 $ 40 $ 55 $ 135 Allagoptera campestris $ 35 Alloschmidtia glabrata $ 35 $ 45 $ 55 $ 85 $ 150 $ 175 Alsmithia longipes $ 35+ $ 55 Aphandra natalia $ 35 $ 55 Archontophoenix Alexandrae $ 55 $ 85 $ 125 inquire Archontophoenix Beatricae $ 20 $ 35 $ 55 $ 125 Archontophoenix $ 25 $ 45 $ 65 $ 100 $ 150- $ 200+ $ 310- 175 350 cunninghamiana Archontophoenix maxima $ 25 $ 30 inquire Archontophoenix maxima (Wash River) Archontophoenix myolaensis $ 25+ $ 30 $ 50 $ 75 $ 125 Archontophoenix purpurea $ 30 $ 25 $ 35 $ 50 $ 85 $ 125 $ 300+ Archontophoenix sp. Archontophoenix tuckerii (peach $ 25+ $ 55 river) Areca alicae $ 45 Areca catechu $ 20 $ 35 $ 45 $ 125 Areca guppyana $ 30 $ 45 Areca ipot $ 45 Areca triandra $ 25 $ 30 $ 95 $ 125 Areca vestiaria $ 25 $ 30-35 $ 35-40 $ 55 $ 85-95 $ 125 Arecastrum romanzoffianum $ 125 Arenga australasica $ 20 $ 30 $ 35 $ 45-55 $ 85 $ 125 Arenga caudata $ 20 $ 30 $ 45 $ 55 $ 75 $ 100 Arenga engleri $ 20 $ 60 $ 35 $ 45 $ 85 $ 125 $ 200 $ 300+ Arenga hastata $ 25 www.junglemusic.net Page 1 of 22 Tree Citrus 25/+ Band$ 1 gal$ 2 gal$ 3/5 gal$ 7 gal$ 15 gal$ 20 gal$ Box$ Species Pot$ Pot$ gal$ Arenga hookeriana inquire Arenga micranthe 'Lhutan' $ 20 inquire Arenga pinnata $ 35 $ 50 $ 85 $ 125 Arenga sp. -

Four New Taxa of Acaulescent Syagrus (Arecaceae) from Brazil
Phytotaxa 188 (1): 001–013 ISSN 1179-3155 (print edition) www.mapress.com/phytotaxa/ PHYTOTAXA Copyright © 2014 Magnolia Press Article ISSN 1179-3163 (online edition) http://dx.doi.org/10.11646/phytotaxa.188.1.1 Four new taxa of acaulescent Syagrus (Arecaceae) from Brazil LARRY R. NOBLICK1, HARRI LORENZI2 & VINICIUS C. SOUZA3 1 Montgomery Botanical Center, Miami, FL, U.S.A.; email: [email protected]; 2Jardim Plantarum, Nova Odessa, SP, Brazil, email: [email protected]; 3Departamento de Ciência Biológicas, ESALQ-USP, Piricicaba, SP, Brazil; email: [email protected] Abstract Three new species and a new subspecies of acaulescent Syagrus palms are described as new to science. These occur in the central western cerrado region of Brazil: Syagrus emasensis and S. menzeliana from southwestern Goiás, S. guimaraesensis from south central Mato Grosso and finally S. graminifolia subsp. cabraliensis from north central Minas Gerais. Keywords: Arecales, Arecoideae, Cocoseae, Palmae Introduction The genus Syagrus currently contains 56 species (Lorenzi et al. 2010, Noblick 2014, Soares et al. 2013). Of these 56 species, 26 are acaulescent (Noblick 2013). The word acaulescent translates as “without a trunk” and a trunk is defined as an above ground stem. While these palms appear to have no above ground stem, they do all have short, subterranean stems. Many of these acaulescent Syagrus are difficult to identify from herbarium material, but leaflet anatomy has been found to be useful in their identification (Glassman 1972, Noblick 2013). The discovery that the acaulescent Syagrus petraea (Mart.) Beccari (1916: 467) was a Bolivian endemic (Noblick et al. 2010), rather than the broadly distributed morphologically variable species that many botanists assumed that it was (Henderson et al. -

(OUV) of the Wet Tropics of Queensland World Heritage Area
Handout 2 Natural Heritage Criteria and the Attributes of Outstanding Universal Value (OUV) of the Wet Tropics of Queensland World Heritage Area The notes that follow were derived by deconstructing the original 1988 nomination document to identify the specific themes and attributes which have been recognised as contributing to the Outstanding Universal Value of the Wet Tropics. The notes also provide brief statements of justification for the specific examples provided in the nomination documentation. Steve Goosem, December 2012 Natural Heritage Criteria: (1) Outstanding examples representing the major stages in the earth’s evolutionary history Values: refers to the surviving taxa that are representative of eight ‘stages’ in the evolutionary history of the earth. Relict species and lineages are the elements of this World Heritage value. Attribute of OUV (a) The Age of the Pteridophytes Significance One of the most significant evolutionary events on this planet was the adaptation in the Palaeozoic Era of plants to life on the land. The earliest known (plant) forms were from the Silurian Period more than 400 million years ago. These were spore-producing plants which reached their greatest development 100 million years later during the Carboniferous Period. This stage of the earth’s evolutionary history, involving the proliferation of club mosses (lycopods) and ferns is commonly described as the Age of the Pteridophytes. The range of primitive relict genera representative of the major and most ancient evolutionary groups of pteridophytes occurring in the Wet Tropics is equalled only in the more extensive New Guinea rainforests that were once continuous with those of the listed area. -

List of Plant Species List of Plant Species
List of plant species List of Plant Species Contents Amendment history .......................................................................................................................... 2 1 Introduction ...................................................................................................................................... 3 1.1 Application ........................................................................................................................... 3 1.2 Relationship with planning scheme ..................................................................................... 3 1.3 Purpose ............................................................................................................................... 3 1.4 Aim ...................................................................................................................................... 3 1.5 Who should use this manual? ............................................................................................. 3 2 Special consideration ....................................................................................................................... 3 3 Variations ......................................................................................................................................... 4 4 Relationship ..................................................................................................................................... 4 Appendix A – Explanatory notes & definitions ....................................................................................... -

The Changing Face of Ethnomedicine in Hiv a Oa, Marquesas Islands a Thesis Submitted To
UNIVERSITY OF HA"/A/I UBRARY NEW PLANTS, NEW DISEASES, NEW PRACTICES: THE CHANGING FACE OF ETHNOMEDICINE IN HIV A OA, MARQUESAS ISLANDS A THESIS SUBMITTED TO THE GRADUATE DIVISION OF THE UNIVERSITY OF HAWArI IN PARTIAL FULFILLMENT OF THE REQUIREMENTS FOR THE DEGREE OF MASTER OF SCIENCE IN BOTANICAL SCIENCE (BOTANY) DECEMBER 2005 By Liloa Makinney Dunn Thesis Committee: Will McClatchey (Chairperson) David Webb Mark Merlin We certify that we have read this thesis and that, in our opinion, it is satisfactory in scope and quality as a thesis for the degree of Master of Science in Botanical Science (Botany). " 1IIIIIIIIIIIIIIIIIIIIIfIlili I 10 002605464 ~_ UNIVERSITY OF HAWAII '" HAWN THESIS COMMITTEE Q111 .H3 no. 4015 II ACKNOWLEDGMENTS I would first like to acknowledge and dedicate this work to the late mayor of Nuku Hiva, Monsieur Lucien" Taaroa" Kimitete, who was the first to open the door to the Marquesas for me. During my first field trip to the Marquesas, both Debora and Lucien Kimitete took me in and made me feel like this was my home. Both he and I planned on collaborating on a book that would document Marquesan Traditional medicine, but his untimely death put those plans on hold. I plan to continue with this work and his vision and would like to dedicate all my work in the Marquesas to his name. I would like to thank my thesis committee members for their guidance through this project, Dr. David Webb, who single handedly got me into the Botany program and who without I wouldn't have done what I have done today, Dr. -

Journal of the International Palm Society Vol. 57(3) Sep. 2013 the INTERNATIONAL PALM SOCIETY, INC
Palms Journal of the International Palm Society Vol. 57(3) Sep. 2013 THE INTERNATIONAL PALM SOCIETY, INC. The International Palm Society Palms (formerly PRINCIPES) Journal of The International Palm Society Founder: Dent Smith The International Palm Society is a nonprofit corporation An illustrated, peer-reviewed quarterly devoted to engaged in the study of palms. The society is inter- information about palms and published in March, national in scope with worldwide membership, and the June, September and December by The International formation of regional or local chapters affiliated with the Palm Society Inc., 9300 Sandstone St., Austin, TX international society is encouraged. Please address all 78737-1135 USA. inquiries regarding membership or information about Editors: John Dransfield, Herbarium, Royal Botanic the society to The International Palm Society Inc., 9300 Gardens, Kew, Richmond, Surrey, TW9 3AE, United Sandstone St., Austin, TX 78737-1135 USA, or by e-mail Kingdom, e-mail [email protected], tel. 44-20- to [email protected], fax 512-607-6468. 8332-5225, Fax 44-20-8332-5278. OFFICERS: Scott Zona, Dept. of Biological Sciences (OE 167), Florida International University, 11200 SW 8 Street, President: Leland Lai, 21480 Colina Drive, Topanga, Miami, Florida 33199 USA, e-mail [email protected], tel. California 90290 USA, e-mail [email protected], 1-305-348-1247, Fax 1-305-348-1986. tel. 1-310-383-2607. Associate Editor: Natalie Uhl, 228 Plant Science, Vice-Presidents: Jeff Brusseau, 1030 Heather Drive, Cornell University, Ithaca, New York 14853 USA, e- Vista, California 92084 USA, e-mail mail [email protected], tel. 1-607-257-0885. -

Watkins Munro Martin Conservatory, Cairns Botanic Gardens
PALM S Dowe & Warmington: Conservatory Vol. 60(1) 2016 Watkins JOHN LESLIE DOWE , Munro Martin James Cook University, Cairns, Queensland, Conservatory, Australia [email protected] Cairns Botanic AND DAVID WARMINGTON Gardens, Cairns Botanic Gardens, Collins Avenue, Edge Hill, Queensland, Queensland, Australia Australia 1. Front view of the Watkins Munro Martin Conservatory, September 2015. Watkins Munro Martin Conservatory in Cairns, Queensland, Australia, was opened in September 2015. The design of the structure uses a Licuala ramsayi leaf as its inspiration. The conservatory houses a substantial collection of rare plants featuring understory tropical palms, aroids, bromeliads, cycads, ferns, Nepenthes , pandans and orchids. On 4 September 2015, the Watkins Munro officially opened by the Mayor of Cairns, Martin Conservatory (Fig. 1) at the Cairns Councilor Bob Manning OAM. The new Botanic Gardens, Queensland, Australia, was conservatory replaces two adjoined structures, PALMS 60(1): 41 –50 41 PALM S Dowe & Warmington: Conservatory Vol. 60(1) 2016 which had previously occupied the site – the visitors. After many cyclones, and the impact Munro Martin Fernery and the George Watkins of tropical conditions on building materials, Orchid House. Both of these structures were the orchid house and fernery were, by the late modest and inadequate to display the gardens’ 1990s, starting to show signs of structural growing collection and provide the best breakdown, which would necessitate their experience to the increasing number of complete rebuilding. Their replacement was 2 (top). Internal view of the Watkins Munro Martin Conservatory, with Licuala cordata in the foreground. 3 (bottom). The roof is supported on steel girders, the longest to 20 m. -

Biosíntesi, Distribució, Acumulació I Funció De La Vitamina E En Llavors: Mecanismes De Control
Biosíntesi, distribució, acumulació i funció de la vitamina E en llavors: mecanismes de control Laura Siles Suárez Aquesta tesi doctoral està subjecta a la llicència Reconeixement- NoComercial – SenseObraDerivada 3.0. Espanya de Creative Commons. Esta tesis doctoral está sujeta a la licencia Reconocimiento - NoComercial – SinObraDerivada 3.0. España de Creative Commons. This doctoral thesis is licensed under the Creative Commons Attribution-NonCommercial- NoDerivs 3.0. Spain License. Barcelona, febrer de 2017 Biosíntesi, distribució, acumulació i funció de la vitamina E en llavors: mecanismes de control Memòria presentada per Laura Siles Suarez per a optar al grau de Doctora per la Universitat de Barcelona. Aquest treball s’emmarca dins el programa de doctorat de BIOLOGIA VEGETAL del Departament de Biologia Evolutiva, Ecologia i Ciències Ambientals (BEECA) de la Facultat de Biologia de la Universitat de Barcelona. El present treball ha estat realitzat al Departament de Biologia Evolutiva, Ecologia i Ciències Ambientals de la Facultat de Biologia (BEECA) de la Universitat de Barcelona sota la direcció de la Dra. Leonor Alegre Batlle i el Dr. Sergi Munné Bosch. Doctoranda: Directora i Codirector de Tesi: Tutora de Tesi: Laura Siles Suarez Dra. Leonor Alegre Batlle Dra. Leonor Alegre Batlle Dr. Sergi Munné Bosch “Mira profundamente en la naturaleza y entonces comprenderás todo mejor”- Albert Einstein. “La creación de mil bosques está en una bellota”-Ralph Waldo Emerson. A mi familia, por apoyarme siempre, y a mis bichejos peludos Índex ÍNDEX AGRAÏMENTS i ABREVIATURES v INTRODUCCIÓ GENERAL 1 Vitamina E 3 1.1.Descobriment i estudi 3 1.2.Estructura química i classes 3 Distribució de la vitamina E 5 Biosíntesi de vitamina E 6 3.1. -

Seed Geometry in the Arecaceae
horticulturae Review Seed Geometry in the Arecaceae Diego Gutiérrez del Pozo 1, José Javier Martín-Gómez 2 , Ángel Tocino 3 and Emilio Cervantes 2,* 1 Departamento de Conservación y Manejo de Vida Silvestre (CYMVIS), Universidad Estatal Amazónica (UEA), Carretera Tena a Puyo Km. 44, Napo EC-150950, Ecuador; [email protected] 2 IRNASA-CSIC, Cordel de Merinas 40, E-37008 Salamanca, Spain; [email protected] 3 Departamento de Matemáticas, Facultad de Ciencias, Universidad de Salamanca, Plaza de la Merced 1–4, 37008 Salamanca, Spain; [email protected] * Correspondence: [email protected]; Tel.: +34-923219606 Received: 31 August 2020; Accepted: 2 October 2020; Published: 7 October 2020 Abstract: Fruit and seed shape are important characteristics in taxonomy providing information on ecological, nutritional, and developmental aspects, but their application requires quantification. We propose a method for seed shape quantification based on the comparison of the bi-dimensional images of the seeds with geometric figures. J index is the percent of similarity of a seed image with a figure taken as a model. Models in shape quantification include geometrical figures (circle, ellipse, oval ::: ) and their derivatives, as well as other figures obtained as geometric representations of algebraic equations. The analysis is based on three sources: Published work, images available on the Internet, and seeds collected or stored in our collections. Some of the models here described are applied for the first time in seed morphology, like the superellipses, a group of bidimensional figures that represent well seed shape in species of the Calamoideae and Phoenix canariensis Hort. ex Chabaud.