In Situ Effects of Elevated CO2 on Plants and Insects
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Biotropika: Journal of Tropical Biology | Vol
E-ISSN 2549-8703 I P-ISSN 2302-7282 BIOTROPIKA Journal of Tropical Biology https://biotropika.ub.ac.id/ Vol. 9 | No. 1 | 2021 | DOI: 10.21776/ub.biotropika.2021.009.01.03 THE ABUNDANCE AND DIVERSITY OF GRASSHOPPER (ORTHOPTERA) IN BATU CITY, EAST JAVA KELIMPAHAN DAN KEANEKARAGAMAN BELALANG (ORTHOPTERA) DI KOTA BATU, JAWA TIMUR Mufti Abrori1)*, Amin Setyo Leksono2), Zulfaidah Penata Gama2) Received : December 14th 2020 ABSTRACT Grasshoppers included in the order Orthoptera in the class of insects. Orthoptera orders Accepted : December 24th 2020 are divided into two parts, which a large suborder Ensifera and Caelifera. Most grasshopper species have a role as herbivores and a good protein source for other animals. Grasshopper abundance and diversity of ecosystems are more stable in a low disorder and the other way around. The factors that affect grasshoppers which environmental factors such as the structure of the vegetation, atmospheric temperature, and relative humidity. Author Affiliation: The purpose of this study to analyze the abundance and diversity of grasshoppers in Batu City, East Java. The research location is in Tahura R. Soerjo Cangar, an agricultural area 1) Master Student, Faculty of in Sumbergondo Village, Coban Talun, and Junrejo District. Measurement of biotic and Mathematics and Natural abiotic factors was carried out at the grasshoppers living locations, and then the data were Sciences, University of Brawijaya analyzed using the Shannon Wiener Diversity index (H'), Importance Value Index (INP), 2) Biology Departmen, Faculty of and Biplot analysis. The results were obtained as 754 individual grasshoppers from the Mathematics and Natural Caelifera suborder. While 201 individuals were found in the Ensifera suborder. -

Catalogue of the Type Specimens Deposited in the Department of Entomology, National Museum, Prague, Czech Republic*
ACTA ENTOMOLOGICA MUSEI NATIONALIS PRAGAE Published 30.iv.2014 Volume 54(1), pp. 399–450 ISSN 0374-1036 http://zoobank.org/urn:lsid:zoobank.org:pub:7479D174-4F1D-4465-9EEA-2BBB5E1FC2A2 Catalogue of the type specimens deposited in the Department of Entomology, National Museum, Prague, Czech Republic* Polyneoptera Lenka MACHÁýKOVÁ & Martin FIKÁýEK Department of Entomology, National Museum in Prague, Kunratice 1, CZ-148 00 Praha 4-Kunratice, Czech Republic & Department of Zoology, Faculty of Sciences, Charles University in Prague, Viniþná 7, CZ-128 43, Praha 2, Czech Republic; e-mails: [email protected]; m¿ [email protected] Abstract. Type specimens from the collection of the polyneopteran insect orders (Dermaptera, Blattodea, Orthoptera, Phasmatodea) deposited in the Department of Entomology, National Museum, Prague are catalogued. We provide precise infor- mation about types of 100 taxa (5 species of Dermaptera, 3 species of Blattodea, 4 species of Phasmatodea, 55 species of Caelifera, and 33 species of Ensifera), including holotypes of 38 taxa. The year of publication of Calliptamus tenuicer- cis anatolicus MaĜan, 1952 and Calliptamus tenuicercis iracus MaĜan, 1952 are corrected. The authorship of the names traditionally ascribed to J. Obenberger is discussed in detail. Only the name Podisma alpinum carinthiacum Obenberger, 1926 is available since the publication by OBENBERGER (1926a). ‘Stenobothrus (Stauroderus) biguttulus ssp. bicolor Charp. 1825’ and ‘Stenobothrus (Stau- roderus) ssp. collinus Karny’ sensu OBENBERGER (1926a,b) refer to Gryllus bicolor Charpentier, 1825 and Stauroderus biguttulus var. collina Karny, 1907, respectively, which both have to be considered available already since their original descriptions by CHARPENTIER (1825) and KARNY (1907). Key words. -

IOBC/WPRS Working Group “Integrated Plant Protection in Fruit
IOBC/WPRS Working Group “Integrated Plant Protection in Fruit Crops” Subgroup “Soft Fruits” Proceedings of Workshop on Integrated Soft Fruit Production East Malling (United Kingdom) 24-27 September 2007 Editors Ch. Linder & J.V. Cross IOBC/WPRS Bulletin Bulletin OILB/SROP Vol. 39, 2008 The content of the contributions is in the responsibility of the authors The IOBC/WPRS Bulletin is published by the International Organization for Biological and Integrated Control of Noxious Animals and Plants, West Palearctic Regional Section (IOBC/WPRS) Le Bulletin OILB/SROP est publié par l‘Organisation Internationale de Lutte Biologique et Intégrée contre les Animaux et les Plantes Nuisibles, section Regionale Ouest Paléarctique (OILB/SROP) Copyright: IOBC/WPRS 2008 The Publication Commission of the IOBC/WPRS: Horst Bathon Luc Tirry Julius Kuehn Institute (JKI), Federal University of Gent Research Centre for Cultivated Plants Laboratory of Agrozoology Institute for Biological Control Department of Crop Protection Heinrichstr. 243 Coupure Links 653 D-64287 Darmstadt (Germany) B-9000 Gent (Belgium) Tel +49 6151 407-225, Fax +49 6151 407-290 Tel +32-9-2646152, Fax +32-9-2646239 e-mail: [email protected] e-mail: [email protected] Address General Secretariat: Dr. Philippe C. Nicot INRA – Unité de Pathologie Végétale Domaine St Maurice - B.P. 94 F-84143 Montfavet Cedex (France) ISBN 978-92-9067-213-5 http://www.iobc-wprs.org Integrated Plant Protection in Soft Fruits IOBC/wprs Bulletin 39, 2008 Contents Development of semiochemical attractants, lures and traps for raspberry beetle, Byturus tomentosus at SCRI; from fundamental chemical ecology to testing IPM tools with growers. -

Bulletin Number / Numéro 2 Entomological Society of Canada Société D’Entomologie Du Canada June / Juin 2008
Volume 40 Bulletin Number / numéro 2 Entomological Society of Canada Société d’entomologie du Canada June / juin 2008 Published quarterly by the Entomological Society of Canada Publication trimestrielle par la Société d’entomologie du Canada ............................................................... .................................................................................................................................................................................................................................................................................................................................. .......................................................................... ........................................................................................................................................................................ ....................... ................................................................................. ................................................. List of contents / Table des matières Volume 40 (2), June / june 2008 Up front / Avant-propos ................................................................................................................49 Moth balls / Boules à mites .............................................................................................................51 Meeting announcements / Réunions futures ..................................................................................52 Dear Buggy / Cher Bibitte ..............................................................................................................53 -

Der Hohen Tauern: Verbreitung, Ökologie, Gemeinschaftsstruktur Und Gefährdung
Wissenschaftliche© Nationalpark Mitteilungen Hohe Tauern, aus download dem unter Nationalpark www.biologiezentrum.at Hohe Tauern ____________________ Bd. 4 (1998): 57-158 ____________________ Die Heuschrecken (Orthoptera: Saltatoria) der Hohen Tauern: Verbreitung, Ökologie, Gemeinschaftsstruktur und Gefährdung Ingeborg P. Illich & Nobert Winding Eingelangt am 21.11.1997 Inhalt 1 Zusammenfassung 58 2 Summary 60 3 Keywords 61 4 Einleitung 61 5 Untersuchungsgebiet und Probeflächen 62 5.1 Untersuchungsgebiete der qualitativen Erfassung 64 5.2 Untersuchungsgebiete der quantitativen Erfassung 64 6 Material und Methoden 65 6.1 Habitaterfassung 65 6.1.1 Ermittlung des Habitatangebotes 65 6.1.2 Ermittlung der Habitatnutzung 65 6.2 Heuschreckenerfassung 66 6.2.1 Untersuchungszeitraum und Witterung 66 6.2.2 Qualitative Erfassung 66 6.2.3 Quantitative Erfassung 67 7 Faunistik und Autökologie - Ergebnisse und Diskussion 67 7.1 Gesamtüberblick 67 7.2 Verbreitung und Ökologie der einzelnen Arten 67 Barbitistes serricauda - Laubholz-Säbelschrecke 67 Tettigonia cantans - Zwitscherschrecke 68 Decticus verrucivorus - Warzenbeißer 70 Platycleis grisea - Graue Beißschrecke 72 Metrioptera roeselii - Roesels Beißschrecke 73 Metrioptera brachyptera - Kurzflügelige Beißschrecke 74 Metrioptera saussuriana - Gebirgs-Beißschrecke 76 Pholidoptera aptera - Alpen-Strauchschrecke 78 Pholidoptera griseoaptera - Gewöhnliche Strauchschrecke 80 Anonconotus alpinus - Alpenschrecke 81 Gryllus campestris -Feldgrille 82 Tetrix subulata - Säbeldornschrecke 83 Tetrix tuerki - -
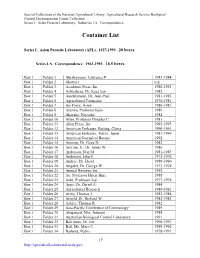
Container List
Special Collections of the National Agricultural Library: Agricultural Research Service Biological Control Documentation Center Collection Series I. Asian Parasite Laboratory. Subseries I.A. Correspondence. Container List Series I. Asian Parasite Laboratory (APL). 1927-1993. 20 boxes. Series I.A. Correspondence. 1963-1993. 16.5 boxes. Box 1 Folder 1 Abrahamson, Lawrence P. 1983-1984 Box 1 Folder 2 Abstract n.d. Box 1 Folder 3 Academic Press, Inc. 1986-1993 Box 1 Folder 4 Achterberg, Dr. Kees van 1982 Box 1 Folder 5 Aeschlimann, Dr. Jean-Paul 1981-1985 Box 1 Folder 6 Agricultural Counselor 1976-1981 Box 1 Folder 7 Air Force, Army 1980-1981 Box 1 Folder 8 Aizawa, Professor Keio 1986 Box 1 Folder 9 Akasaka, Naoyuki 1982 Box 1 Folder 10 Allen, Professor Douglas C. 1981 Box 1 Folder 11 Allen Press, Inc. 1992-1993 Box 1 Folder 12 American Embassy, Beijing, China 1990-1991 Box 1 Folder 13 American Embassy, Tokyo, Japan 1981-1990 Box 1 Folder 14 American Journal of Botany 1992 Box 1 Folder 15 Amman, Dr. Gene D. 1982 Box 1 Folder 16 Amrine, Jr., Dr. James W. 1986 Box 1 Folder 17 Anderson, Don M. 1981-1983 Box 1 Folder 18 Anderson, John F. 1975-1976 Box 1 Folder 19 Andres, Dr. Lloyd 1989-1990 Box 1 Folder 20 Angalet, Dr. George W. 1973-1978 Box 1 Folder 21 Annual Reviews Inc. 1992 Box 1 Folder 22 Ao, Professor Hsien-Bine 1988 Box 1 Folder 23 Aoki, Professor Joji 1977-1978 Box 1 Folder 24 Apps, Dr. Darrel A. 1984 Box 1 Folder 25 Agricultural Research 1980-1981 Box 1 Folder 26 Army, Thomas J. -

Draft Carpathian Red List of Forest Habitats
CARPATHIAN RED LIST OF FOREST HABITATS AND SPECIES CARPATHIAN LIST OF INVASIVE ALIEN SPECIES (DRAFT) PUBLISHED BY THE STATE NATURE CONSERVANCY OF THE SLOVAK REPUBLIC 2014 zzbornik_cervenebornik_cervene zzoznamy.inddoznamy.indd 1 227.8.20147.8.2014 222:36:052:36:05 © Štátna ochrana prírody Slovenskej republiky, 2014 Editor: Ján Kadlečík Available from: Štátna ochrana prírody SR Tajovského 28B 974 01 Banská Bystrica Slovakia ISBN 978-80-89310-81-4 Program švajčiarsko-slovenskej spolupráce Swiss-Slovak Cooperation Programme Slovenská republika This publication was elaborated within BioREGIO Carpathians project supported by South East Europe Programme and was fi nanced by a Swiss-Slovak project supported by the Swiss Contribution to the enlarged European Union and Carpathian Wetlands Initiative. zzbornik_cervenebornik_cervene zzoznamy.inddoznamy.indd 2 115.9.20145.9.2014 223:10:123:10:12 Table of contents Draft Red Lists of Threatened Carpathian Habitats and Species and Carpathian List of Invasive Alien Species . 5 Draft Carpathian Red List of Forest Habitats . 20 Red List of Vascular Plants of the Carpathians . 44 Draft Carpathian Red List of Molluscs (Mollusca) . 106 Red List of Spiders (Araneae) of the Carpathian Mts. 118 Draft Red List of Dragonfl ies (Odonata) of the Carpathians . 172 Red List of Grasshoppers, Bush-crickets and Crickets (Orthoptera) of the Carpathian Mountains . 186 Draft Red List of Butterfl ies (Lepidoptera: Papilionoidea) of the Carpathian Mts. 200 Draft Carpathian Red List of Fish and Lamprey Species . 203 Draft Carpathian Red List of Threatened Amphibians (Lissamphibia) . 209 Draft Carpathian Red List of Threatened Reptiles (Reptilia) . 214 Draft Carpathian Red List of Birds (Aves). 217 Draft Carpathian Red List of Threatened Mammals (Mammalia) . -

ARTICULATA 1993 8(2): 1 -22 SYSTEMATIK to the Knowledge Of
ZOBODAT - www.zobodat.at Zoologisch-Botanische Datenbank/Zoological-Botanical Database Digitale Literatur/Digital Literature Zeitschrift/Journal: Articulata - Zeitschrift der Deutschen Gesellschaft für Orthopterologie e.V. DGfO Jahr/Year: 1993 Band/Volume: 8_2_1993 Autor(en)/Author(s): Storozhenko Sergey Artikel/Article: To the knowledge of the tribe Melanoplini (Orthoptera, Acrididae: Catantopinae) of the Eastern Palearctlca 1-22 Deutschen Gesellschaft für Orthopterologie e.V.; download http://www.dgfo-articulata.de/ ARTICULATA 1993 8(2): 1 -22 SYSTEMATIK To the knowledge of the tribe Melanoplini (Orthoptera, Acrididae: Catantopinae) of the Eastern Palearctlca Sergey Storozhenko Abstract Data on the grasshoppers of the tribe Melanoplini SCUDDER, 1897 (= Podismini JACOBSON, 1905 = Parapodisminae INOUE, 1985, syn. n) of Eastern Palearctica are given. Podisma kanoi sp. n. and Podisma sapporensis ashibetsuensis ssp. n. from Japan are described. The new synonyms are established: Rhinopodisma MISTSHENKO, 1954 = Aserratus HUANG, 1981, syn. n., Sinopodisma CHANG, 1940 = Pedopodisma ZHENG, 1980, syn. n., Parapodisma MISTSHENKO, 1947 = Pseudoparapodisma INOUE, 1985, syn. n., Monopterus FISCHER-WALDHEIM, 1846 = Bohemanella RAMME, 1951, syn.n. Tribe Melanoplini SCUDDER, 1897 Type genus: Melanoplus STAL, 1873. Notes The tribe Melanoplini was established by S.SCUDDER (1897) as a group Melanopli. JACOBSON (1905) proposed Podismini as a new name for this group. In the most modem classification the position of tribe Melanoplini is following: MISTSHENKO (1952) considered it as a tribe Podismini of subfamily Catanto pinae (Acrididae); UVAROV (1966) as Catantopinae (without division on tribes); DIRSH (1975) as subfamily Podisminae of family Catantopidae; HARZ (1975) as tribe Podismini of subfamily Catantopinae (Acrididae); VICKERY & KEVAN (1983) as subfamily Melanoplinae of family Acrididae with two tribes (Melanoplini and Podismini) and YIN (1984) as subfamily Podisminae of family Oedipodidae. -

A Karyogram Study on Eighteen Species of Japanese Acrididae (Orthoptera) (With 36 Figures)
Title A Karyogram Study on Eighteen Species of Japanese Acrididae (Orthoptera) (With 36 Figures) Author(s) MOMMA, Eizi Citation 北海道帝國大學理學部紀要, 9(1), 59-69 Issue Date 1943-07 Doc URL http://hdl.handle.net/2115/27051 Type bulletin (article) File Information 9(1)_P59-69.pdf Instructions for use Hokkaido University Collection of Scholarly and Academic Papers : HUSCAP A Karyogram. Study on Eighteen Species of Japanese Acrididae (Orthoptera)l) By Eizi Momma Zoological Institute, Faculty of Science, Hokkaido Imperial University, Sapporo (W1~th 36 Figures) During these forty years the cytological investigations of the Acridian grasshoppers have been extensively carried out by a number of investigators, and considerable contributions have been devoted to the advance of animal cytology. The orthopteran insects, especially those of the Acrididae, have proved very favourable as material for the study of chromosomes, on account of the fact that their chromosomes are large in size having clear morphological features and are relatively low in number. Over one hundred of species have been cytologically investigated since the pioneer work of McClung ('00) in this field, demonstrating many valuable and • important facts and their bearing on problems of general cytology. One of the important matters confronting the students of Acridian chromosomes is to determine the interrelationships of the chromosome complexes in the different species. The extensive studies by McClung and his colleagues have established a great uniformity and constancy of the chromosome numbers in the Acrididae. Efforts made by these investigators greatly served to homologize individual chromosomes in closely related genera and species. Recently Ramachandra Rao (,37) has made a comparative study of the chromosomes in eight genera of Pyrgomorphinae (Acrididae), in view of finding the chromosomal relationship exist ing among the members of this subfamily. -

Towards Classical Biological Control of Leek Moth
____________________________________________________________________________ Ateyyat This project seeks to provide greater coherence for the biocontrol knowledge system for regulators and researchers; create an open access information source for biocontrol re- search of agricultural pests in California, which will stimulate greater international knowl- edge sharing about agricultural pests in Mediterranean climates; and facilitate the exchange of information through a cyberinfrastructure among government regulators, and biocontrol entomologists and practitioners. It seeks broader impacts through: the uploading of previ- ously unavailable data being made openly accessible; the stimulation of greater interaction between the biological control regulation, research, and practitioner community in selected Mediterranean regions; the provision of more coherent and useful information to enhance regulatory decisions by public agency scientists; a partnership with the IOBC to facilitate international data sharing; and progress toward the ultimate goal of increasing the viability of biocontrol as a reduced risk pest control strategy. No Designated Session Theme BIOLOGY OF CIRROSPILUS INGENUUS GAHAN (HYMENOPTERA: EULOPHIDAE), AN ECTOPARASITOID OF THE CITRUS LEAFMINER, PHYLLOCNISTIS CITRELLA STAINTON (LEPIDOPTERA: GRACILLARIIDAE) ON LEMON 99 Mazen A. ATEYYAT Al-Shoubak University College, Al-Balqa’ Applied University, P.O. Box (5), Postal code 71911, Al-Shawbak, Jordan [email protected] The citrus leafminer (CLM), Phyllocnistis citrella Stainton (Lepidoptera: Gracillariidae) in- vaded the Jordan Valley in 1994 and was able to spread throughout Jordan within a few months of its arrival. It was the most common parasitoid from 1997 to 1999 in the Jordan Valley. An increase in the activity of C. ingenuus was observed in autumn and the highest number of emerged C. ingenuus adults was in November 1999. -

New Cockroaches (Dictyoptera: Blattina) from Baltic Amber, with Description of a New Genus and Species: Stegoblatta Irmgardgroeh
Proceedings of the Zoological Institute RAS Vol. 316, No. 3, 2012, рр. 193–202 УДК 595.722 NEW COCKROACHES (DICTYOPTERA: BLATTINA) FROM BALTIC AMBER, WITH THE DESCRIPTION OF A NEW GENUS AND SPECIES: STEGOBLATTA IRMGARDGROEHNI L.N. Anisyutkin1* and C. Gröhn2 1Zoological Institute of the Russian Academy of Sciences, Universitetskaya Emb. 1, 199034 Saint Petersburg, Russia; e-mail: [email protected] 2Bünebüttler Weg 7, D-21509 Glinde/Hamburg, Germany; e-mail: [email protected] ABSTRACT A new genus and species of cockroaches, Stegoblatta irmgardgroehni gen. et sp. nov. is described from Baltic Amber. The taxonomic position of the new genus is discussed and it is concluded that it belongs to the family Blaberidae. The male of Paraeuthyrrapha groehni (Corydiidae, Euthyrrhaphinae) is described for the first time. Key words: Baltic Amber, Blaberidae, cockroaches, Dictyoptera, Paraeuthyrrapha groehni, Stegoblatta irmgard- groehni gen. et sp. nov. НОВЫЕ ТАРАКАНЫ (DICTYOPTERA: BLATTINA) ИЗ БАЛТИЙСКОГО ЯНТАРЯ, С ОПИСАНИЕМ НОВОГО РОДА И ВИДА: STEGOBLATTA IRMGARDGROEHNI Л.Н. Анисюткин1* и К. Грён2 1Зоологический институт Российской академии наук, Университетская наб. 1, 199034 Санкт-Петербург, Россия; e-mail: [email protected] 2Bünebüttler Weg 7, D-21509 Glinde/Hamburg, Germany; e-mail: [email protected] РЕЗЮМЕ Новый род и вид тараканов (Stegoblatta irmgardgroehni gen. et sp. nov.) описывается из балтийского янта- ря. Обсуждается таксономическое положение нового рода, предположительно отнесенного к семейству Blaberidae. Впервые описывается самец Paraeuthyrrhapha groehni (Corydiidae, Euthyrrhaphinae). Ключевые слова: балтийский янтарь, Blaberidae, тараканы, Dictyoptera, Paraeuthyrrapha groehni, Stegoblatta irmgardgroehni gen. et sp. nov. INTRODUCTION Wichard 2010). The cockroach fauna from Baltic amber is more or less similar to the modern one in Baltic amber is one of the most famous sources of taxa composition (Shelford 1910, 1911; Weitshat fossil insects. -

A Proteomic Approach for Studying Insect Phylogeny: CAPA Peptides of Ancient Insect Taxa (Dictyoptera, Blattoptera) As a Test Case
BMC Evolutionary Biology BioMed Central Research article Open Access A proteomic approach for studying insect phylogeny: CAPA peptides of ancient insect taxa (Dictyoptera, Blattoptera) as a test case Steffen Roth1,3, Bastian Fromm1, Gerd Gäde2 and Reinhard Predel*1 Address: 1Institute of Zoology, University of Jena, Erbertstrasse 1, D-07743 Jena, Germany, 2Zoology Department, University of Cape Town, Rondebosch 7701, South Africa and 3Institute of Biology, University of Bergen, Bergen N-5020, Norway Email: Steffen Roth - [email protected]; Bastian Fromm - [email protected]; Gerd Gäde - [email protected]; Reinhard Predel* - [email protected] * Corresponding author Published: 3 March 2009 Received: 6 October 2008 Accepted: 3 March 2009 BMC Evolutionary Biology 2009, 9:50 doi:10.1186/1471-2148-9-50 This article is available from: http://www.biomedcentral.com/1471-2148/9/50 © 2009 Roth et al; licensee BioMed Central Ltd. This is an Open Access article distributed under the terms of the Creative Commons Attribution License (http://creativecommons.org/licenses/by/2.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited. Abstract Background: Neuropeptide ligands have to fit exactly into their respective receptors and thus the evolution of the coding regions of their genes is constrained and may be strongly conserved. As such, they may be suitable for the reconstruction of phylogenetic relationships within higher taxa. CAPA peptides of major lineages of cockroaches (Blaberidae, Blattellidae, Blattidae, Polyphagidae, Cryptocercidae) and of the termite Mastotermes darwiniensis were chosen to test the above hypothesis. The phylogenetic relationships within various groups of the taxon Dictyoptera (praying mantids, termites and cockroaches) are still highly disputed.