The Evolution of the Social and Genetic Mating System
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

678.973.2437 770.493.8862 AAS Goes to Colombia
April 2010 Volume XXXVI, Issue 4 ATLANTA AUDUBON SOCIETY AAS Goes to Colombia INSIDE By Ted Reissing GOS Guided Tour..................2 Now that the narco-terrorists have been brought under control, birders are flocking back to Colombia. First Time Birders ................2 With almost 10% of the world’s bird species (more than twice as many as can be found in the entire U.S.) and about 75 endemics, this country is a natural target for listers. In addition, the top bird Annual Report ......................3 conservation group in the country, ProAves, has developed a series of 15 preserves to protect specific birds and created lodging facilities to house visitors. Because of all these developments, AAS put Field Notes - January ..........4 together a trip to do some serious birding in Colombia and the results of this outing are highlighted Field Trips.............................5 here. Delta flies directly from Atlanta to Bogotá daily and the four-hour A Million Thanks..................6 flight arrives just after 9 PM (there is no time change when we are on standard time). If you do start in Colombia’s capital city, an Volunteer Opportunities.......6 early morning visit to a local park can reveal eight to 10 good lifers Conservation Days...............6 including the endemic Bogotá Rail. From there it is usually about an eight-hour motor trip to one of the major preserves. For this tour Merritt Island.......................7 we chose El Paujil, the prime site for the critically endangered Blue-billed Curassow. Very few outsiders have seen this bird in the Bird Journal ........................7 wild, but after a couple of days of climbing steep trails in 95°F and Blue-billed Curassow Sculpting Birds....................8 Photographer: ProAves 90% humidity, we were fortunate to see two birds that flew directly over our heads. -

21 Sep 2018 Lists of Victims and Hosts of the Parasitic
version: 21 Sep 2018 Lists of victims and hosts of the parasitic cowbirds (Molothrus). Peter E. Lowther, Field Museum Brood parasitism is an awkward term to describe an interaction between two species in which, as in predator-prey relationships, one species gains at the expense of the other. Brood parasites "prey" upon parental care. Victimized species usually have reduced breeding success, partly because of the additional cost of caring for alien eggs and young, and partly because of the behavior of brood parasites (both adults and young) which may directly and adversely affect the survival of the victim's own eggs or young. About 1% of all bird species, among 7 families, are brood parasites. The 5 species of brood parasitic “cowbirds” are currently all treated as members of the genus Molothrus. Host selection is an active process. Not all species co-occurring with brood parasites are equally likely to be selected nor are they of equal quality as hosts. Rather, to varying degrees, brood parasites are specialized for certain categories of hosts. Brood parasites may rely on a single host species to rear their young or may distribute their eggs among many species, seemingly without regard to any characteristics of potential hosts. Lists of species are not the best means to describe interactions between a brood parasitic species and its hosts. Such lists do not necessarily reflect the taxonomy used by the brood parasites themselves nor do they accurately reflect the complex interactions within bird communities (see Ortega 1998: 183-184). Host lists do, however, offer some insight into the process of host selection and do emphasize the wide variety of features than can impact on host selection. -

Colombia Trip Report Santa Marta Extension 25Th to 30Th November 2014 (6 Days)
RBT Colombia: Santa Marta Extension Trip Report - 2014 1 Colombia Trip Report Santa Marta Extension 25th to 30th November 2014 (6 days) Buffy Hummingbird by Clayton Burne Trip report compiled by tour leader: Clayton Burne RBT Colombia: Santa Marta Extension Trip Report - 2014 2 Our Santa Marta extension got off to a flying start with some unexpected birding on the first afternoon. Having arrived in Barranquilla earlier than expected, we wasted no time and headed out to the nearby Universidad del Norte – one of the best places to open our Endemics account. It took only a few minutes to find Chestnut- winged Chachalaca, and only a few more to obtain excellent views of a number of these typically localised birds. A fabulous welcome meal was then had on the 26th floor of our city skyscraper hotel! An early start the next day saw us leaving the city of Barranquilla for the nearby scrub of Caño Clarín. Our account opened quickly with a female Sapphire-throated Hummingbird followed by many Russet-throated Puffbirds. A Chestnut-winged Chachalaca by Clayton Burne White-tailed Nightjar was the surprise find of the morning. We added a number of typical species for the area including Caribbean Hornero, Scaled Dove, Green-and-rufous, Green and Ringed Kingfishers, Red-crowned, Red-rumped and Spot-breasted Woodpeckers, Stripe-backed and Bicolored Wrens, as well as Black-crested Antshrike. Having cleared up the common stuff, we headed off to Isla de Salamanca, a mangrove reserve that plays host to another very scarce endemic, the Sapphire-bellied Hummingbird. More good luck meant that the very first bird we saw after climbing out of the vehicle was the targeted bird itself. -

Bogota, the Magdalena Valley & Santa
® field guides BIRDING TOURS WORLDWIDE [email protected] • 800•728•4953 ITINERARY COLOMBIA: BOGOTA, THE MAGDALENA VALLEY & SANTA MARTA January 9-24, 2021 One of the range-restricted species we’ll seek on this tour is the Rusty-breasted Antpitta. These tiny ground-dwellers are found in the mountains of northern Colombia and Venezuela. We’ll look for this skulker in the Sierra Nevada de Santa Marta. Photograph by guide Jesse Fagan. We include here information for those interested in the 2021 Field Guides Colombia: Bogota, the Magdalena Valley & Santa Marta tour: ¾ a general introduction to the tour ¾ a description of the birding areas to be visited on the tour ¾ an abbreviated daily itinerary with some indication of the nature of each day’s birding outings These additional materials will be made available to those who register for the tour: ¾ an annotated list of the birds recorded on a previous year’s Field Guides trip to the area, with comments by guide(s) on notable species or sightings (may be downloaded from our web site) ¾ a detailed information bulletin with important logistical information and answers to questions regarding accommodations, air arrangements, clothing, currency, customs and immigration, documents, health precautions, and personal items ¾ a reference list ¾ a Field Guides checklist for preparing for and keeping track of the birds we see on the tour ¾ after the conclusion of the tour, a list of birds seen on the tour 1900+ species. Subtract the species recorded on that archipelago off Central America (San Andres, if you care), and Colombia is still ahead of Brazil and Peru, let alone our most popular South American destination, Ecuador, which is several hundred species behind. -

Buckbird Journeys
BUCKBIRD JOURNEYS YEMEN and SOCOTRA Tuesday 8 – Sunday 18 November 2007 Participants Louise Augustine (LA), Hugh Buck (HB), David Daniels (DD), David Hoddinott (DH), Pearl Jordan (PJ), Werner Suter (WS), David Bradford (DB – Socotra only) This trip was designed to try for all the Southwest Arabia and Socotra endemics in a relatively brief time scale. It also represented an opportunity for regional listers to add an enticing selection of “African” species only entering Asia in Southwest Arabia and a selection of “Asian” species found in Africa rarely outside of Socotra. That the trip was successful on all counts is a tribute to Yousuf Mohageb (YM) of Arabian Eco- Tours in Sana’a who designed, with HB, the itinerary, accompanied us throughout Yemen and knew all the best birding spots, Ali his redoubtable co-driver and Ahmed Saeid Suliman (AS) on Socotra whose knowledge and love of all things on his island home is second to none. Our multinational group (three Americans, two Brits, a South African and a Swiss) kept to the pace admirably, provided exceptional spotting skills and, in WS, some wonderful photographic documentation and memories. Day by Day Thursday 8 November HB, DD and DH meet up at Dubai International Airport for the short Emirates Airlines flight to Sana’a where they are met by YM, Ali in traditional Yemeni costume and LA and PJ who have arrived the previous evening. Under the high bright sun, which will be the norm for the next 10 days, we traverse Sana’a’s sprawling outskirts to the Funduk Arabia Felix, an interesting hotel made up a several old traditional houses right at the edge of the historic and UNESCO rated old town. -

Troglodytidae Species Tree
Troglodytidae I Rock Wren, Salpinctes obsoletus Canyon Wren, Catherpes mexicanus Sumichrast’s Wren, Hylorchilus sumichrasti Nava’s Wren, Hylorchilus navai Salpinctinae Nightingale Wren / Northern Nightingale-Wren, Microcerculus philomela Scaly-breasted Wren / Southern Nightingale-Wren, Microcerculus marginatus Flutist Wren, Microcerculus ustulatus Wing-banded Wren, Microcerculus bambla ?Gray-mantled Wren, Odontorchilus branickii Odontorchilinae Tooth-billed Wren, Odontorchilus cinereus Bewick’s Wren, Thryomanes bewickii Carolina Wren, Thryothorus ludovicianus Thrush-like Wren, Campylorhynchus turdinus Stripe-backed Wren, Campylorhynchus nuchalis Band-backed Wren, Campylorhynchus zonatus Gray-barred Wren, Campylorhynchus megalopterus White-headed Wren, Campylorhynchus albobrunneus Fasciated Wren, Campylorhynchus fasciatus Cactus Wren, Campylorhynchus brunneicapillus Yucatan Wren, Campylorhynchus yucatanicus Giant Wren, Campylorhynchus chiapensis Bicolored Wren, Campylorhynchus griseus Boucard’s Wren, Campylorhynchus jocosus Spotted Wren, Campylorhynchus gularis Rufous-backed Wren, Campylorhynchus capistratus Sclater’s Wren, Campylorhynchus humilis Rufous-naped Wren, Campylorhynchus rufinucha Pacific Wren, Nannus pacificus Winter Wren, Nannus hiemalis Eurasian Wren, Nannus troglodytes Zapata Wren, Ferminia cerverai Marsh Wren, Cistothorus palustris Sedge Wren, Cistothorus platensis ?Merida Wren, Cistothorus meridae ?Apolinar’s Wren, Cistothorus apolinari Timberline Wren, Thryorchilus browni Tepui Wren, Troglodytes rufulus Troglo dytinae Ochraceous -

The Marine Biodiversity and Fisheries Catches of the Pitcairn Island Group
The Marine Biodiversity and Fisheries Catches of the Pitcairn Island Group THE MARINE BIODIVERSITY AND FISHERIES CATCHES OF THE PITCAIRN ISLAND GROUP M.L.D. Palomares, D. Chaitanya, S. Harper, D. Zeller and D. Pauly A report prepared for the Global Ocean Legacy project of the Pew Environment Group by the Sea Around Us Project Fisheries Centre The University of British Columbia 2202 Main Mall Vancouver, BC, Canada, V6T 1Z4 TABLE OF CONTENTS FOREWORD ................................................................................................................................................. 2 Daniel Pauly RECONSTRUCTION OF TOTAL MARINE FISHERIES CATCHES FOR THE PITCAIRN ISLANDS (1950-2009) ...................................................................................... 3 Devraj Chaitanya, Sarah Harper and Dirk Zeller DOCUMENTING THE MARINE BIODIVERSITY OF THE PITCAIRN ISLANDS THROUGH FISHBASE AND SEALIFEBASE ..................................................................................... 10 Maria Lourdes D. Palomares, Patricia M. Sorongon, Marianne Pan, Jennifer C. Espedido, Lealde U. Pacres, Arlene Chon and Ace Amarga APPENDICES ............................................................................................................................................... 23 APPENDIX 1: FAO AND RECONSTRUCTED CATCH DATA ......................................................................................... 23 APPENDIX 2: TOTAL RECONSTRUCTED CATCH BY MAJOR TAXA ............................................................................ -
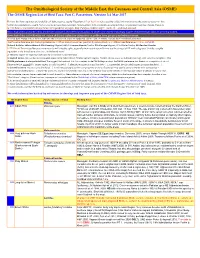
OSME List V3.4 Passerines-2
The Ornithological Society of the Middle East, the Caucasus and Central Asia (OSME) The OSME Region List of Bird Taxa: Part C, Passerines. Version 3.4 Mar 2017 For taxa that have unproven and probably unlikely presence, see the Hypothetical List. Red font indicates either added information since the previous version or that further documentation is sought. Not all synonyms have been examined. Serial numbers (SN) are merely an administrative conveninence and may change. Please do not cite them as row numbers in any formal correspondence or papers. Key: Compass cardinals (eg N = north, SE = southeast) are used. Rows shaded thus and with yellow text denote summaries of problem taxon groups in which some closely-related taxa may be of indeterminate status or are being studied. Rows shaded thus and with white text contain additional explanatory information on problem taxon groups as and when necessary. A broad dark orange line, as below, indicates the last taxon in a new or suggested species split, or where sspp are best considered separately. The Passerine Reference List (including References for Hypothetical passerines [see Part E] and explanations of Abbreviated References) follows at Part D. Notes↓ & Status abbreviations→ BM=Breeding Migrant, SB/SV=Summer Breeder/Visitor, PM=Passage Migrant, WV=Winter Visitor, RB=Resident Breeder 1. PT=Parent Taxon (used because many records will antedate splits, especially from recent research) – we use the concept of PT with a degree of latitude, roughly equivalent to the formal term sensu lato , ‘in the broad sense’. 2. The term 'report' or ‘reported’ indicates the occurrence is unconfirmed. -

AOU Classification Committee – North and Middle America
AOU Classification Committee – North and Middle America Proposal Set 2015-A 21 Jan 2015 No. Page Title 01 02 Revise the classification of the Pipridae 02 08 Add Bicolored Wren Campylorhynchus griseus to the Main List 03 11 Move Dusky Pigeon Patagioenas goodsoni from the Appendix to the Main List 04 14 Revise the classification of the Psittaciformes 05 19 Split Pterodroma heraldica and P. atrata from Herald Petrel P. arminjoniana 06 26 Transfer American Tree Sparrow Spizella arborea to Spizelloides 07 28 Split Passerina pallidior from Painted Bunting P. ciris 08 32 Split Toxostoma arenicola from LeConte’s Thrasher T. lecontei 09 35 Correct the scientific names of (a) Leptotila cassini and (b) Amazilia saucerrottei 10 37 Split Laysan Honeycreeper from Apapane Himatione sanguinea and change its specific epithet to fraithii 11 40 Split Newell’s Shearwater Puffinus newelli from Townsend’s Shearwater P. auricularis, and consider Rapa Shearwater P. myrtae as a species separate from P. newelli 12 44 Correct the citation for Pterodroma solandri 2015-A-1 N&MA Classification Committee pp. 423-426 Revise the classification of the Pipridae Background: Our current classification of the Pipridae is as follows: Corapipo altera Chiroxiphia lanceolata Chiroxiphia linearis Xenopipo holochlora Dixiphia pipra Ceratopipra mentalis Ceratopipra erythrocephala Manacus candei Manacus aurantiacus Manacus vitellinus Lepidothrix coronata New information: Ohlson et al. (2013) investigated relationships within the family using DNA sequence data from three nuclear introns and one mitochondrial gene (ND2). They sampled all genera and most species. I have pasted in a screen grab of their tree below. Their results are largely consistent with those of previous studies except for the polyphyly of Chloropipo, members of which are in three parts of the tree. -
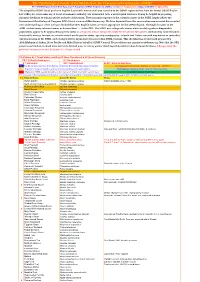
Simplified-ORL-2019-5.1-Final.Pdf
The Ornithological Society of the Middle East, the Caucasus and Central Asia (OSME) The OSME Region List of Bird Taxa, Part F: Simplified OSME Region List (SORL) version 5.1 August 2019. (Aligns with ORL 5.1 July 2019) The simplified OSME list of preferred English & scientific names of all taxa recorded in the OSME region derives from the formal OSME Region List (ORL); see www.osme.org. It is not a taxonomic authority, but is intended to be a useful quick reference. It may be helpful in preparing informal checklists or writing articles on birds of the region. The taxonomic sequence & the scientific names in the SORL largely follow the International Ornithological Congress (IOC) List at www.worldbirdnames.org. We have departed from this source when new research has revealed new understanding or when we have decided that other English names are more appropriate for the OSME Region. The English names in the SORL include many informal names as denoted thus '…' in the ORL. The SORL uses subspecific names where useful; eg where diagnosable populations appear to be approaching species status or are species whose subspecies might be elevated to full species (indicated by round brackets in scientific names); for now, we remain neutral on the precise status - species or subspecies - of such taxa. Future research may amend or contradict our presentation of the SORL; such changes will be incorporated in succeeding SORL versions. This checklist was devised and prepared by AbdulRahman al Sirhan, Steve Preddy and Mike Blair on behalf of OSME Council. Please address any queries to [email protected]. -

AMNH-Scientific-Publications-2014
AMERICAN MUSEUM OF NATURAL HISTORY Fiscal Year 2014 Scientific Publications Division of Anthropology 2 Division of Invertebrate Zoology 11 Division of Paleontology 28 Division of Physical Sciences 39 Department of Earth and Planetary Sciences and Department of Astrophysics Division of Vertebrate Zoology Department of Herpetology 58 Department of Ichthyology 62 Department of Mammalogy 65 Department of Ornithology 78 Center for Biodiversity and Conservation 91 Sackler Institute for Comparative Genomics 99 DIVISION OF ANTHROPOLOGY Berwick, R.C., M.D. Hauser, and I. Tattersall. 2013. Neanderthal language? Just-so stories take center stage. Frontiers in Psychology 4, article 671. Blair, E.H., and Thomas, D.H. 2014. The Guale uprising of 1597: an archaeological perspective from Mission Santa Catalina de Guale (Georgia). In L.M. Panich and T.D. Schneider (editors), Indigenous Landscapes and Spanish Missions: New Perspectives from Archaeology and Ethnohistory: 25–40. Tucson: University of Arizona Press. Charpentier, V., A.J. de Voogt, R. Crassard, J.-F. Berger, F. Borgi, and A. Al- Ma’shani. 2014. Games on the seashore of Salalah: the discovery of mancala games in Dhofar, Sultanate of Oman. Arabian Archaeology and Epigraphy 25: 115– 120. Chowns, T.M., A.H. Ivester, R.L. Kath, B.K. Meyer, D.H. Thomas, and P.R. Hanson. 2014. A New Hypothesis for the Formation of the Georgia Sea Islands through the Breaching of the Silver Bluff Barrier and Dissection of the Ancestral Altamaha-Ogeechee Drainage. Abstract, 63rd Annual Meeting, Geological Society of America, Southeastern Section, April 10–11, 2014. 2 DeSalle, R., and I. Tattersall. 2014. Mr. Murray, you lose the bet. -

The Birds of Africa, Comprising All the Species Which Occur in The
: ^rpl, THE BIRDS OF AFRICA, COMPRISING ALL THE SPECIES WHICH OCCUR ETHIOPIAN REGION. BY &C. G. E. SHELLEY, F.Z.S., F.R.G.S., (late gkenadier guaeds), aitthor of "a handbook to the birds of egypt,' "a monograph of the sunbirds," etc. VOL. III. LONDON PUBLISHED FOR THE AUTHOR EY CAVENDISH SQUARE, W. E. H. POETER, 7, PEINCES STEEET, 1902. liw^<J^ ? SEP 18 1902/ ^fiiii CONTENTS. vi. LIST OF PLATES—VOL. IIL Plate XV., Ordei- I. PASSERIFORMES. Suborder II. OSCINES. Section II. ALAUD^. Family VII. MOTACILLID.^. Genus III. MACRONYX. The Long-clawR, as Dr. Bovvdler Sharpe calls them, in the " Birds of South Africa," may be described as heavily-built Pipits. Their feet are extremely large, the hind claw long, and also the tarsus, so that the outstretched feet extend well beyond the end of the tail, although the tail is not abnormally short. This character, together with the bright colouring of the throat, and often of the breast, render the species of this genus easily recognisable. Anatomically they are Pipits. Type. iMacronyx, Swains. Zool. .Journ, iii. p. .344 (1817) .... M. capensis. KEY TO THE SPECIES. n. Five outer pairs of tail-feathers with white ends ; throat and centre of breast reddish orange capensis. 2. h. Four outer pairs of tail-feathers with white ends ; no shade of red on the throat or breast. «^. Throat and some of the breast bright lemon yellow. «2. Smaller ; wing less than 4 inches ; upper croceus. parts paler ; less brown on the breast V b^. Larger; wing 4-2 to 4-4; upper parts darker ; more brown on the breast .