BONNER ZOOLOGISCHE MONOGRAPHIEN, Nr
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Costa Rica 2020
Sunrise Birding LLC COSTA RICA TRIP REPORT January 30 – February 5, 2020 Photos: Talamanca Hummingbird, Sunbittern, Resplendent Quetzal, Congenial Group! Sunrise Birding LLC COSTA RICA TRIP REPORT January 30 – February 5, 2020 Leaders: Frank Mantlik & Vernon Campos Report and photos by Frank Mantlik Highlights and top sightings of the trip as voted by participants Resplendent Quetzals, multi 20 species of hummingbirds Spectacled Owl 2 CR & 32 Regional Endemics Bare-shanked Screech Owl 4 species Owls seen in 70 Black-and-white Owl minutes Suzy the “owling” dog Russet-naped Wood-Rail Keel-billed Toucan Great Potoo Tayra!!! Long-tailed Silky-Flycatcher Black-faced Solitaire (& song) Rufous-browed Peppershrike Amazing flora, fauna, & trails American Pygmy Kingfisher Sunbittern Orange-billed Sparrow Wayne’s insect show-and-tell Volcano Hummingbird Spangle-cheeked Tanager Purple-crowned Fairy, bathing Rancho Naturalista Turquoise-browed Motmot Golden-hooded Tanager White-nosed Coati Vernon as guide and driver January 29 - Arrival San Jose All participants arrived a day early, staying at Hotel Bougainvillea. Those who arrived in daylight had time to explore the phenomenal gardens, despite a rain storm. Day 1 - January 30 Optional day-trip to Carara National Park Guides Vernon and Frank offered an optional day trip to Carara National Park before the tour officially began and all tour participants took advantage of this special opportunity. As such, we are including the sightings from this day trip in the overall tour report. We departed the Hotel at 05:40 for the drive to the National Park. En route we stopped along the road to view a beautiful Turquoise-browed Motmot. -

Antbird Guilds in the Lowland Caribbean Rainforest of Southeast Nicaragua1
The Condor 102:7X4-794 0 The Cooper Ornithological Society 2000 ANTBIRD GUILDS IN THE LOWLAND CARIBBEAN RAINFOREST OF SOUTHEAST NICARAGUA1 MARTIN L. CODY Department of OrganismicBiology, Ecology and Evolution, Universityof California, Los Angeles, CA 90095-1606, e-mail: [email protected] Abstract. Some 20 speciesof antbirdsoccur in lowland Caribbeanrainforest in southeast Nicaragua where they form five distinct guilds on the basis of habitat preferences,foraging ecology, and foraging behavior. Three guilds are habitat-based,in Edge, Forest, and Gaps within forest; two are behaviorally distinct, with species of army ant followers and those foraging within mixed-species flocks. The guilds each contain 3-6 antbird species. Within guilds, species are segregatedby body size differences between member species, and in several guilds are evenly spaced on a logarithmic scale of body mass. Among guilds, the factors by which adjacent body sizes differ vary between 1.25 and 1.75. Body size differ- ences may be related to differences in preferred prey sizes, but are influenced also by the density of the vegetation in which each speciescustomarily forages. Resumen. Unas 20 especies de aves hormiguerasviven en el bosque tropical perenni- folio, surestede Nicaragua, donde se forman cinquo gremios distinctos estribando en pre- ferencias de habitat, ecologia y comportamiento de las costumbresde alimentacion. Las diferenciasentre las varias especiesson cuantificadaspor caractaristicasde1 ambiente vegetal y por la ecologia y comportamientode la alimentaci6n, y usadospara definir cinco grupos o gremios (“guilds”). Tres gremios se designanpor las relacionesde habitat: edge (margen), forest (selva), y gaps (aberturasadentro la selva); dos mas por comportamiento,partidarios de army ants (hormigasarmadas) y mixed-speciesflocks (forrejando en bandadasde especies mexcladas). -
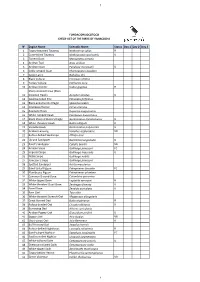
N° English Name Scientific Name Status Day 1
1 FUNDACIÓN JOCOTOCO CHECK-LIST OF THE BIRDS OF YANACOCHA N° English Name Scientific Name Status Day 1 Day 2 Day 3 1 Tawny-breasted Tinamou Nothocercus julius R 2 Curve-billed Tinamou Nothoprocta curvirostris U 3 Torrent Duck Merganetta armata 4 Andean Teal Anas andium 5 Andean Guan Penelope montagnii U 6 Sickle-winged Guan Chamaepetes goudotii 7 Cattle Egret Bubulcus ibis 8 Black Vulture Coragyps atratus 9 Turkey Vulture Cathartes aura 10 Andean Condor Vultur gryphus R Sharp-shinned Hawk (Plain- 11 breasted Hawk) Accipiter striatus U 12 Swallow-tailed Kite Elanoides forficatus 13 Black-and-chestnut Eagle Spizaetus isidori 14 Cinereous Harrier Circus cinereus 15 Roadside Hawk Rupornis magnirostris 16 White-rumped Hawk Parabuteo leucorrhous 17 Black-chested Buzzard-Eagle Geranoaetus melanoleucus U 18 White-throated Hawk Buteo albigula R 19 Variable Hawk Geranoaetus polyosoma U 20 Andean Lapwing Vanellus resplendens VR 21 Rufous-bellied Seedsnipe Attagis gayi 22 Upland Sandpiper Bartramia longicauda R 23 Baird's Sandpiper Calidris bairdii VR 24 Andean Snipe Gallinago jamesoni FC 25 Imperial Snipe Gallinago imperialis U 26 Noble Snipe Gallinago nobilis 27 Jameson's Snipe Gallinago jamesoni 28 Spotted Sandpiper Actitis macularius 29 Band-tailed Pigeon Patagoienas fasciata FC 30 Plumbeous Pigeon Patagioenas plumbea 31 Common Ground-Dove Columbina passerina 32 White-tipped Dove Leptotila verreauxi R 33 White-throated Quail-Dove Zentrygon frenata U 34 Eared Dove Zenaida auriculata U 35 Barn Owl Tyto alba 36 White-throated Screech-Owl Megascops -

Tinamiformes – Falconiformes
LIST OF THE 2,008 BIRD SPECIES (WITH SCIENTIFIC AND ENGLISH NAMES) KNOWN FROM THE A.O.U. CHECK-LIST AREA. Notes: "(A)" = accidental/casualin A.O.U. area; "(H)" -- recordedin A.O.U. area only from Hawaii; "(I)" = introducedinto A.O.U. area; "(N)" = has not bred in A.O.U. area but occursregularly as nonbreedingvisitor; "?" precedingname = extinct. TINAMIFORMES TINAMIDAE Tinamus major Great Tinamou. Nothocercusbonapartei Highland Tinamou. Crypturellus soui Little Tinamou. Crypturelluscinnamomeus Thicket Tinamou. Crypturellusboucardi Slaty-breastedTinamou. Crypturellus kerriae Choco Tinamou. GAVIIFORMES GAVIIDAE Gavia stellata Red-throated Loon. Gavia arctica Arctic Loon. Gavia pacifica Pacific Loon. Gavia immer Common Loon. Gavia adamsii Yellow-billed Loon. PODICIPEDIFORMES PODICIPEDIDAE Tachybaptusdominicus Least Grebe. Podilymbuspodiceps Pied-billed Grebe. ?Podilymbusgigas Atitlan Grebe. Podicepsauritus Horned Grebe. Podicepsgrisegena Red-neckedGrebe. Podicepsnigricollis Eared Grebe. Aechmophorusoccidentalis Western Grebe. Aechmophorusclarkii Clark's Grebe. PROCELLARIIFORMES DIOMEDEIDAE Thalassarchechlororhynchos Yellow-nosed Albatross. (A) Thalassarchecauta Shy Albatross.(A) Thalassarchemelanophris Black-browed Albatross. (A) Phoebetriapalpebrata Light-mantled Albatross. (A) Diomedea exulans WanderingAlbatross. (A) Phoebastriaimmutabilis Laysan Albatross. Phoebastrianigripes Black-lootedAlbatross. Phoebastriaalbatrus Short-tailedAlbatross. (N) PROCELLARIIDAE Fulmarus glacialis Northern Fulmar. Pterodroma neglecta KermadecPetrel. (A) Pterodroma -

Ultimate Bolivia Tour Report 2019
Titicaca Flightless Grebe. Swimming in what exactly? Not the reed-fringed azure lake, that’s for sure (Eustace Barnes) BOLIVIA 8 – 29 SEPTEMBER / 4 OCTOBER 2019 LEADER: EUSTACE BARNES Bolivia, indeed, THE land of parrots as no other, but Cotingas as well and an astonishing variety of those much-loved subfusc and generally elusive denizens of complex uneven surfaces. Over 700 on this tour now! 1 BirdQuest Tour Report: Ultimate Bolivia 2019 www.birdquest-tours.com Blue-throated Macaws hoping we would clear off and leave them alone (Eustace Barnes) Hopefully, now we hear of colourful endemic macaws, raucous prolific birdlife and innumerable elusive endemic denizens of verdant bromeliad festooned cloud-forests, vast expanses of rainforest, endless marshlands and Chaco woodlands, each ringing to the chorus of a diverse endemic avifauna instead of bleak, freezing landscapes occupied by impoverished unhappy peasants. 2 BirdQuest Tour Report: Ultimate Bolivia 2019 www.birdquest-tours.com That is the flowery prose, but Bolivia IS that great destination. The tour is no longer a series of endless dusty journeys punctuated with miserable truck-stop hotels where you are presented with greasy deep-fried chicken and a sticky pile of glutinous rice every day. The roads are generally good, the hotels are either good or at least characterful (in a good way) and the food rather better than you might find in the UK. The latter perhaps not saying very much. Palkachupe Cotinga in the early morning light brooding young near Apolo (Eustace Barnes). That said, Bolivia has work to do too, as its association with that hapless loser, Che Guevara, corruption, dust and drug smuggling still leaves the country struggling to sell itself. -

COLOMBIA 2019 Ned Brinkley Departments of Vaupés, Chocó, Risaralda, Santander, Antioquia, Magdalena, Tolima, Atlántico, La Gu
COLOMBIA 2019 Ned Brinkley Departments of Vaupés, Chocó, Risaralda, Santander, Antioquia, Magdalena, Tolima, Atlántico, La Guajira, Boyacá, Distrito Capital de Bogotá, Caldas These comments are provided to help independent birders traveling in Colombia, particularly people who want to drive themselves to birding sites rather than taking public transportation and also want to book reservations directly with lodgings and reserves rather than using a ground agent or tour company. Many trip reports provide GPS waypoints for navigation. I used GoogleEarth/ Maps, which worked fine for most locations (not for El Paujil reserve). I paid $10/day for AT&T to hook me up to Claro, Movistar, or Tigo through their Passport program. Others get a local SIM card so that they have a Colombian number (cheaper, for sure); still others use GooglePhones, which provide connection through other providers with better or worse success, depending on the location in Colombia. For transportation, I used a rental 4x4 SUV to reach places with bad roads but also, in northern Colombia, a subcompact rental car as far as Minca (hiked in higher elevations, with one moto-taxi to reach El Dorado lodge) and for La Guajira. I used regular taxis on few occasions. The only roads to sites for Fuertes’s Parrot and Yellow-eared Parrot could not have been traversed without four-wheel drive and high clearance, and this is important to emphasize: vehicles without these attributes would have been useless, or become damaged or stranded. Note that large cities in Colombia (at least Medellín, Santa Marta, and Cartagena) have restrictions on driving during rush hours with certain license plate numbers (they base restrictions on the plate’s final numeral). -

A Comprehensive Species-Level Molecular Phylogeny of the New World
YMPEV 4758 No. of Pages 19, Model 5G 2 December 2013 Molecular Phylogenetics and Evolution xxx (2013) xxx–xxx 1 Contents lists available at ScienceDirect Molecular Phylogenetics and Evolution journal homepage: www.elsevier.com/locate/ympev 5 6 3 A comprehensive species-level molecular phylogeny of the New World 4 blackbirds (Icteridae) a,⇑ a a b c d 7 Q1 Alexis F.L.A. Powell , F. Keith Barker , Scott M. Lanyon , Kevin J. Burns , John Klicka , Irby J. Lovette 8 a Department of Ecology, Evolution and Behavior, and Bell Museum of Natural History, University of Minnesota, 100 Ecology Building, 1987 Upper Buford Circle, St. Paul, MN 9 55108, USA 10 b Department of Biology, San Diego State University, San Diego, CA 92182, USA 11 c Barrick Museum of Natural History, University of Nevada, Las Vegas, NV 89154, USA 12 d Fuller Evolutionary Biology Program, Cornell Lab of Ornithology, Cornell University, 159 Sapsucker Woods Road, Ithaca, NY 14950, USA 1314 15 article info abstract 3117 18 Article history: The New World blackbirds (Icteridae) are among the best known songbirds, serving as a model clade in 32 19 Received 5 June 2013 comparative studies of morphological, ecological, and behavioral trait evolution. Despite wide interest in 33 20 Revised 11 November 2013 the group, as yet no analysis of blackbird relationships has achieved comprehensive species-level sam- 34 21 Accepted 18 November 2013 pling or found robust support for most intergeneric relationships. Using mitochondrial gene sequences 35 22 Available online xxxx from all 108 currently recognized species and six additional distinct lineages, together with strategic 36 sampling of four nuclear loci and whole mitochondrial genomes, we were able to resolve most relation- 37 23 Keywords: ships with high confidence. -

678.973.2437 770.493.8862 AAS Goes to Colombia
April 2010 Volume XXXVI, Issue 4 ATLANTA AUDUBON SOCIETY AAS Goes to Colombia INSIDE By Ted Reissing GOS Guided Tour..................2 Now that the narco-terrorists have been brought under control, birders are flocking back to Colombia. First Time Birders ................2 With almost 10% of the world’s bird species (more than twice as many as can be found in the entire U.S.) and about 75 endemics, this country is a natural target for listers. In addition, the top bird Annual Report ......................3 conservation group in the country, ProAves, has developed a series of 15 preserves to protect specific birds and created lodging facilities to house visitors. Because of all these developments, AAS put Field Notes - January ..........4 together a trip to do some serious birding in Colombia and the results of this outing are highlighted Field Trips.............................5 here. Delta flies directly from Atlanta to Bogotá daily and the four-hour A Million Thanks..................6 flight arrives just after 9 PM (there is no time change when we are on standard time). If you do start in Colombia’s capital city, an Volunteer Opportunities.......6 early morning visit to a local park can reveal eight to 10 good lifers Conservation Days...............6 including the endemic Bogotá Rail. From there it is usually about an eight-hour motor trip to one of the major preserves. For this tour Merritt Island.......................7 we chose El Paujil, the prime site for the critically endangered Blue-billed Curassow. Very few outsiders have seen this bird in the Bird Journal ........................7 wild, but after a couple of days of climbing steep trails in 95°F and Blue-billed Curassow Sculpting Birds....................8 Photographer: ProAves 90% humidity, we were fortunate to see two birds that flew directly over our heads. -

Peru: from the Cusco Andes to the Manu
The critically endangered Royal Cinclodes - our bird-of-the-trip (all photos taken on this tour by Pete Morris) PERU: FROM THE CUSCO ANDES TO THE MANU 26 JULY – 12 AUGUST 2017 LEADERS: PETE MORRIS and GUNNAR ENGBLOM This brand new itinerary really was a tour of two halves! For the frst half of the tour we really were up on the roof of the world, exploring the Andes that surround Cusco up to altitudes in excess of 4000m. Cold clear air and fantastic snow-clad peaks were the order of the day here as we went about our task of seeking out a number of scarce, localized and seldom-seen endemics. For the second half of the tour we plunged down off of the mountains and took the long snaking Manu Road, right down to the Amazon basin. Here we traded the mountainous peaks for vistas of forest that stretched as far as the eye could see in one of the planet’s most diverse regions. Here, the temperatures rose in line with our ever growing list of sightings! In all, we amassed a grand total of 537 species of birds, including 36 which provided audio encounters only! As we all know though, it’s not necessarily the shear number of species that counts, but more the quality, and we found many high quality species. New species for the Birdquest life list included Apurimac Spinetail, Vilcabamba Thistletail, Am- pay (still to be described) and Vilcabamba Tapaculos and Apurimac Brushfnch, whilst other montane goodies included the stunning Bearded Mountaineer, White-tufted Sunbeam the critically endangered Royal Cinclodes, 1 BirdQuest Tour Report: Peru: From the Cusco Andes to The Manu 2017 www.birdquest-tours.com These wonderful Blue-headed Macaws were a brilliant highlight near to Atalaya. -

21 Sep 2018 Lists of Victims and Hosts of the Parasitic
version: 21 Sep 2018 Lists of victims and hosts of the parasitic cowbirds (Molothrus). Peter E. Lowther, Field Museum Brood parasitism is an awkward term to describe an interaction between two species in which, as in predator-prey relationships, one species gains at the expense of the other. Brood parasites "prey" upon parental care. Victimized species usually have reduced breeding success, partly because of the additional cost of caring for alien eggs and young, and partly because of the behavior of brood parasites (both adults and young) which may directly and adversely affect the survival of the victim's own eggs or young. About 1% of all bird species, among 7 families, are brood parasites. The 5 species of brood parasitic “cowbirds” are currently all treated as members of the genus Molothrus. Host selection is an active process. Not all species co-occurring with brood parasites are equally likely to be selected nor are they of equal quality as hosts. Rather, to varying degrees, brood parasites are specialized for certain categories of hosts. Brood parasites may rely on a single host species to rear their young or may distribute their eggs among many species, seemingly without regard to any characteristics of potential hosts. Lists of species are not the best means to describe interactions between a brood parasitic species and its hosts. Such lists do not necessarily reflect the taxonomy used by the brood parasites themselves nor do they accurately reflect the complex interactions within bird communities (see Ortega 1998: 183-184). Host lists do, however, offer some insight into the process of host selection and do emphasize the wide variety of features than can impact on host selection. -

Birding in Bolivia Trip Report November 1991–January 1992
Birding in Bolivia Trip Report November 1991–January 1992 Rolf A. de By1 Sjoerd Mayer Ankrot 19 Casilla 5067 7523 LH Enschede Santa Cruz The Netherlands Bolivia September 30, 1993 1Email address: [email protected] Introduction From 20th November, 1991 until 18th January, 1992 the authors were birding in interesting areas in Bolivia. For the first four weeks Jeroen de By served as our photographer. Our prime interest was to find and study some of the highland forest birds of this country, as well as to have a general birding trip through the country. It was not contemplated to be a ‘twitching trip’, meaning that our prime purpose was not just to find as many bird species as possible. Still, due to the amazing number of birds found in the country, we identified some 466 species in just eight weeks. (For the twitcher planning a trip of this length: some good preparation seems to be a guarantee of a 600+ trip.) This trip report consists of several parts. The introduction serves as a place for general information. There is a section named “Site report” that describes the major birding areas that we visited and the birds observed there. The “Trapping report” finally, describes the information relevant to the birds that we captured. In the site reports we have included maps at some points for a better understanding of the local situation. These maps are not to scale, and should be used only for general reference purposes! Typically, foot paths will be exaggerated and rivers and main roads will be drawn to a smaller scale. -

Creación De Un Manual Interpretativo Para El Buen
CARRERA ADMINISTRACIÓN TURÍSTICA Y HOTELERA CREACIÓN DE UN MANUAL INTERPRETATIVO SOBRE EL BUEN AVISTAMIENTO DE AVES EN LA RESERVA YANACOCHA UBICADA EN LA PARROQUIA DE NONO CANTÓN QUITO PROVINCIA PICHINCHA CON EL PROPÓSITO DE DAR A CONOCER EL AVITURISMO Proyecto de investigación previo a la obtención de título de tecnólogo en Administración Turística y Hotelera Autora: Amanda Estefania Tituaña Espinosa Tutor: Ing. Ximena Almeida Quito, Diciembre 2018 i Declaratoria Declaro que la investigación es absolutamente original, autentica, personal, que se han citado las fuentes correspondientes y en su ejecución se respetaron las disposiciones legales que protegen los derechos de autor vigentes. Las ideas, doctrinas resultados y conclusiones a los que he llegado son de mi absoluta responsabilidad. Amanda Estefania Tituaña Espinosa CC 1750804161 CREACIÓN DE UN MANUAL INTERPRETATIVO SOBRE EL BUEN AVISTAMIENTO DE AVES EN LA RESERVA YANACOCHA UBICADA EN LA PARROQUIA DE NONO CANTON QUITO PROVINCIA PICHINCHA CON EL PROPOSITO DE DAR A CONOCER EL AVITURISMO ii Licencia De Uso No Comercial Yo, Amanda Estefania Tituaña Espinosa portadora de la cedula de ciudadanía asignada Con el No. 175080416-1 de conformidad con lo establecido en el Artículo 110 del Código de Economía Social de los Conocimientos, la Creación y la Innovación (INGENIOS) que dice: “En el caso de las obras creadas en centros educativos,universidades,escuelas politécnicas, institutos superiores tecnicos,tecnólogos, pedagógicos, de arte y los conservatorios superiores , e institutos públicos de investigación como resultado de su actividad académica o de investigación tales como trabajos de titulación, proyectos de investigación o innovación, articulo académico , u otros análogos , sin perjuicio de que pueda existir relación de dependencia , la titularidad de los derechos patrimoniales corresponderá a los autores .