Cinereus Shrew
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Occurrences of Small Mammal Species in a Mixedgrass Prairie in Northwestern North Dakota
University of Nebraska - Lincoln DigitalCommons@University of Nebraska - Lincoln The Prairie Naturalist Great Plains Natural Science Society 6-2007 Occurrences of Small Mammal Species in a Mixedgrass Prairie in Northwestern North Dakota, Robert K. Murphy Richard A. Sweitzer John D. Albertson Follow this and additional works at: https://digitalcommons.unl.edu/tpn Part of the Biodiversity Commons, Botany Commons, Ecology and Evolutionary Biology Commons, Natural Resources and Conservation Commons, Systems Biology Commons, and the Weed Science Commons This Article is brought to you for free and open access by the Great Plains Natural Science Society at DigitalCommons@University of Nebraska - Lincoln. It has been accepted for inclusion in The Prairie Naturalist by an authorized administrator of DigitalCommons@University of Nebraska - Lincoln. NOTES OCCURRENCES OF SMALL MAMMAL SPECIES IN A MIXED-GRASS PRAIRIE IN NORTHWESTERN NORTH DAKOTA -- Documentation is limited for many species of vertebrates in the northern Great Plains, particularly northwestern North Dakota (Bailey 1926, Hall 1981). Here we report relative abundances of small « 450 g) species of mammals that were captured incidental to surveys of amphibians and reptiles at Lostwood National Wildlife Refuge (LNWR) in northwestern North Dakota from 1985 to 1987 and 1999 to 2000. Our records include a modest range extension for one species. We also comment on relationships of small mammals on the refuge to vegetation changes associated with fire and grazing disturbances. LNWR encompassed 109 km2 of rolling to hilly moraine in Burke and Mountrail counties, North Dakota (48°37'N; 102°27'W). The area was mostly a native needlegrass-wheatgrass prairie (Stipa-Agropyron; Coupland 1950) inter spersed with numerous wetlands 'x = 40 basins/km2) and patches of quaking aspen trees (Populus tremuloides; x = 0.4 ha/patch and 4.8 patches/km2; 1985 data in Murphy 1993:23), with a semi-arid climate. -

Proceedings of the Third Annual Northeastern Forest Insect Work Conference
Proceedings of the Third Annual Northeastern Forest Insect Work Conference New Haven, Connecticut 17 -19 February 1970 U.S. D.A. FOREST SERVICE RESEARCH PAPER NE-194 1971 NORTHEASTERN FOREST EXPERIMENT STATION, UPPER DARBY, PA. FOREST SERVICE, U.S. DEPARTMENT OF AGRICULTURE WARREN T. DOOLITTLE, DIRECTOR Proceedings of the Third Annual Northeastern Forest Insect Work Conference CONTENTS INTRODUCTION-Robert W. Campbell ........................... 1 TOWARD INTEGRATED CONTROL- D. L,Collifis ...............................................................................2 POPULATION QUALITY- 7 David E. Leonard ................................................................... VERTEBRATE PREDATORS- C. H. Backner ............................................................................2 1 INVERTEBRATE PREDATORS- R. I. Sailer ..................................................................................32 PATHOGENS-Gordon R. Stairs ...........................................45 PARASITES- W.J. Tamock and I. A. Muldrew .......................................................................... 59 INSECTICIDES-Carroll Williams and Patrick Shea .............................................................................. 88 INTEGRATED CONTROL, PEST MANAGEMENT, OR PROTECTIVE POPULATION MANAGEMENT- R. W. Stark ..............................................................................1 10 INTRODUCTION by ROBERT W. CAMPBELL, USDA Forest Service, Northeastern Forest Experiment Station, Hamden, Connecticut. ANYPROGRAM of integrated control is -

Download Vol. 13, No. 4
BULLETIN OF THE FLORIDA STATE MUSEUM BIOLOGICAL SCIENCES Volume 13 Number 4 THE MAMMAL FAUNA OF SCHULZE CAVE, F EDWARDS COUNTY, TEXAS Walter W. Dalquest, Edward Roth, and Frank Judd 354\ UNIVERSITY OF FLORIDA Gainesville 1969 Numbers of the BULLETIN OF THE FLORIDA STATE MUSEUM are pub- lished at irregular intervals. Volumes contain about 800 pages and are not necessarily completed in any one calendar year. WALTER AUF'FENBERG, Managing Editor OLIVER L. AUSTIN, JR., Editor Consultants for this isstie: THOMAS PATTON ELIZABETH WING Communications concerning purchase or exchange of the publication and all manuscripts should be addressed to the Managing Editor of the Bulletin, Florida State Museum, Seagle Building, Gainesville, Florida 32601. Published June 8, 1969 Price for this issue $.90 THE MAMMAL FAUNA OF SCHULZE CAVE, EDWARDS COUNTY, TEXAS WALTER W. DALQUEST, EDWARD ROTH, AND FRANK JUDD SYNOPSIS: Vertebrate remains from two levels in Schulze Cave, Edwards County, Texas, are analyzed. The younger materials probably date from ca. 5,000 B.P. to 8,800 B.P. The fauna is essentially modern, but the absence of the armadillo collared peccary, ringtail, and rock squirrel is thought to be significant. The older materials probably date from ca. 11,000 B.P. to 8;000 B.P. The mam- malian fauna of these Pleistocene sediments includes 62 species, of which 8 are extinct, 19 are not now regident on the Edwards Plateau, and 40 still live in the general area of the cave. Three species have not previously been reported from Pleistocene deposits in Texas: vagrant shrew, eastern chipmunk, and western jumping mouse. -

Northern Short−Tailed Shrew (Blarina Brevicauda)
FIELD GUIDE TO NORTH AMERICAN MAMMALS Northern Short−tailed Shrew (Blarina brevicauda) ORDER: Insectivora FAMILY: Soricidae Blarina sp. − summer coat Credit: painting by Nancy Halliday from Kays and Wilson's Northern Short−tailed Shrews have poisonous saliva. This enables Mammals of North America, © Princeton University Press them to kill mice and larger prey and paralyze invertebrates such as (2002) snails and store them alive for later eating. The shrews have very limited vision, and rely on a kind of echolocation, a series of ultrasonic "clicks," to make their way around the tunnels and burrows they dig. They nest underground, lining their nests with vegetation and sometimes with fur. They do not hibernate. Their day is organized around highly active periods lasting about 4.5 minutes, followed by rest periods that last, on average, 24 minutes. Population densities can fluctuate greatly from year to year and even crash, requiring several years to recover. Winter mortality can be as high as 90 percent in some areas. Fossils of this species are known from the Pliocene, and fossils representing other, extinct species of the genus Blarina are even older. Also known as: Short−tailed Shrew, Mole Shrew Sexual Dimorphism: Males may be slightly larger than females. Length: Range: 118−139 mm Weight: Range: 18−30 g http://www.mnh.si.edu/mna 1 FIELD GUIDE TO NORTH AMERICAN MAMMALS Least Shrew (Cryptotis parva) ORDER: Insectivora FAMILY: Soricidae Least Shrews have a repertoire of tiny calls, audible to human ears up to a distance of only 20 inches or so. Nests are of leaves or grasses in some hidden place, such as on the ground under a cabbage palm leaf or in brush. -

Appendix D: Species Lists
Appendix D: Species Lists Appendix D: Species Lists Mammal Species List for Rice Lake NWR ........................................................................................................ 81 Butterfly and Moth Species List, Rice Lake NWR ............................................................................................ 83 Dragonfly and Damselfly Species List, Rice Lake NWR ................................................................................... 86 Amphibian and Reptile Species List, Rice Lake NWR ...................................................................................... 90 Fish Species List, Rice Lake NWR and the Sandstone Unit of Rice Lake NWR ............................................... 91 Mollusk and Crustacean Species List, Rice Lake NWR and the Sandstone Unit of Rice Lake NWR .............. 92 Bird Species List, Rice Lake NWR ..................................................................................................................... 93 Sandstone Unit of Rice Lake NWR Bird Species List ..................................................................................... 103 Rice Lake NWR Tree and Shrub Species List ................................................................................................. 112 Vegetation Species List, Rice Lake NWR ....................................................................................................... 116 Rice Lake NWR and Mille Lacs NWR Comprehensive Conservation Plan 79 Appendix D: Species Lists Mammal Species List for Rice -

Checklist of Amphibians, Reptiles, Birds and Mammals of New York
CHECKLIST OF AMPHIBIANS, REPTILES, BIRDS AND MAMMALS OF NEW YORK STATE Including Their Legal Status Eastern Milk Snake Moose Blue-spotted Salamander Common Loon New York State Artwork by Jean Gawalt Department of Environmental Conservation Division of Fish and Wildlife Page 1 of 30 February 2019 New York State Department of Environmental Conservation Division of Fish and Wildlife Wildlife Diversity Group 625 Broadway Albany, New York 12233-4754 This web version is based upon an original hard copy version of Checklist of the Amphibians, Reptiles, Birds and Mammals of New York, Including Their Protective Status which was first published in 1985 and revised and reprinted in 1987. This version has had substantial revision in content and form. First printing - 1985 Second printing (rev.) - 1987 Third revision - 2001 Fourth revision - 2003 Fifth revision - 2005 Sixth revision - December 2005 Seventh revision - November 2006 Eighth revision - September 2007 Ninth revision - April 2010 Tenth revision – February 2019 Page 2 of 30 Introduction The following list of amphibians (34 species), reptiles (38), birds (474) and mammals (93) indicates those vertebrate species believed to be part of the fauna of New York and the present legal status of these species in New York State. Common and scientific nomenclature is as according to: Crother (2008) for amphibians and reptiles; the American Ornithologists' Union (1983 and 2009) for birds; and Wilson and Reeder (2005) for mammals. Expected occurrence in New York State is based on: Conant and Collins (1991) for amphibians and reptiles; Levine (1998) and the New York State Ornithological Association (2009) for birds; and New York State Museum records for terrestrial mammals. -

SEC 1987 Non-Game Animals in the Skagit Valley of Dritish Columbia
E~JIO Non-game animals in the Skagit Valley of Dritish Columbia : literature r eview and research directions La urie L . Kremsat er SEC 1987 /fl Non-game animals in the Skagit Valley of British Columbia: literature review and research directions Laurie L. Kremsater prepared for Ministry of Environment and Parks January 1987 -1- ACKNOWLEDGEMENTS r I am indebted to Fred Bunnell for reviewing this manuscript. ^ Thanks to Dave Dunbar for supplying valuable information and necessary literature. r To the Vertebrate Zoology Division of the British Columbia f Provincial Museum, particularly Mike McNall and Dave Nagorsen, I am grateful for the access provided me to the wildlife record f" scheme and the reprint collections. Thanks also to Richard C Cannings of the Vertebrate Museum of the Department of Zoology of the University of British Columbia for access to wildlife sighting records. H Thanks to Anthea Farr and Barry Booth for their first hand observations of non-game wildlife in the Skagit. Thanks also to Fred Bunnell, Andrew Harcombe, Alton Harestad, Brian Nyberg, and f' Steve Wetmore for their input into potential research directions for non-game animals in the Skagit Valley. ( f I ABSTRACT Extant data on the abundance, distribution, and diversity o non-game animals of the Skagit Valley (specifically, small mammals, non-game avifauna, cavity-using species, and waterfowl) are reviewed. Principal sources include Slaney (1973), wildlife records of the British Columbia Provincial Museum Vertebrate Division, University of British Columbia Vertebrate Museum records, and naturalist field trip records. Totals of 24 small mammal and 186 avian species have been recorded in the Skagit Valley to date. -

Final Plan Chippewa Plains-Pine Moraines and Outwash Plains-SFRMP
APPENDIX L Terrestrial, Vertebrate Species List Chippewa Plains / Pine Moraines and Outwash Plains ECS Subsections a Species Common Name: Are standardized nomenclature for GAP protocol uses through NatureServe and its related searchable plant, animal and ecological communities database called NatureServe Explorer (2002) located at: http://www.natureserveexplorer.org. b Resident Status: R=Regular resident as Breeding, Nesting, or Migratory (acceptable record exists in at least eight of the past 10 years); PR=Permanent Resident (exists year- round). c State Legal Status: E=State Endangered; T=State Threatened; SC=State Species of Special Concern; BG=Big Game; SG=Small Game; F=Furbearer; MW=Migratory Waterfowl; UB=Unprotected Bird; PB=Protected Bird; PWA=Protected Wild Animal; UWA=Unprotected Wild Animal. d Federal Legal Status: T=Federal Threatened; E=Federal Endangered; P=Federal Protection by Migratory Bird Treaty Act and/or Bald Eagle Protection Act and/or CITES. e ECS Subsection Resident Status: B=Minnesota breeding record exists for the species; P=Presence known or predicted, as year around resident; M=Spring or fall migrant, non- breeder; SV= Summer visitor, non-breeder; WV=Winter visitor, non-breeder; A=Absent; (L)=Limited distribution within ECS Subsection. * Species of Greatest Conservation Need Terrestrial Vertebrate Species List Minnesota DNR-Division of Fish and Wildlife - Wildlife Resources Assessment ProjectA ECS Subsectione Pine State Federal Moraines/ Resident Legal Legal Outwash Chippewa Common Namea Scientific Name Statusb -
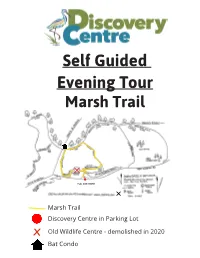
Self Guided Marsh Trail Loop Tour
Self Guided Evening Tour Marsh Trail YOU ARE HERE Marsh Trail Discovery Centre in Parking Lot x Old Wildlife Centre - demolished in 2020 Bat Condo WELCOME TO THE MARSH TRAIL As you embark on the Marsh Trail you will have a good view of the wetlands of the Creston Valley Wildlife Management Area (CVWMA). The CVWMA is a 17 000 acre wetland that is in- ternationally recognized as a Ramsar site and nationally as an Important Bird Area of Canada. The area supports an amazing amount of biodiversity. There are over 300 bird, close to 60 mam- mal, 17 fish, 6 reptile and 6 amphibian species that have been recorded in the area. Plus, there are thousands of invertebrate and plant species! Did you know? • Since 1800, 20 million hectares (~15%) of Canada’s wetlands have been filled in and lost to development. Near major cities and towns, 70% of wetlands have been lost. • When one hectare of wetlands is converted to agricultural land, between 1 and 19 tonnes of carbon dioxide (a greenhouse gas) are emitted to the atmosphere per year. • Most wildlife in the province use wetland habitat at some point in their life cycle. • Wetlands cover about 6% of the land in BC. • One hectare of wetland can store between 9 and 14 million liters of water. NORTH AMERICA’S LARGEST RODENT As you walk along the channel and make your way across the Beaver Boulevard Bridge, stop to admire the dams that our friendly neighborhood beaver has been hard at work creating. These semi-aquatic mammals are often referred to as ‘ecosys- tem engineers’ because of their dams. -

Predicted Shifts in Small Mammal Distributions and Biodiversity in the Altered Future Environment of Alaska: an Open Access Data and Machine Learning Perspective
RESEARCH ARTICLE Predicted Shifts in Small Mammal Distributions and Biodiversity in the Altered Future Environment of Alaska: An Open Access Data and Machine Learning Perspective A. P. Baltensperger*, F. Huettmann EWHALE Lab, Department of Biology and Wildlife, Institute of Arctic Biology, University of Alaska Fairbanks, Fairbanks, Alaska, United States of America * [email protected] Abstract Climate change is acting to reallocate biomes, shift the distribution of species, and alter OPEN ACCESS community assemblages in Alaska. Predictions regarding how these changes will affect the biodiversity and interspecific relationships of small mammals are necessary to pro-actively Citation: Baltensperger AP, Huettmann F (2015) Predicted Shifts in Small Mammal Distributions and inform conservation planning. We used a set of online occurrence records and machine Biodiversity in the Altered Future Environment of learning methods to create bioclimatic envelope models for 17 species of small mammals Alaska: An Open Access Data and Machine Learning (rodents and shrews) across Alaska. Models formed the basis for sets of species-specific Perspective. PLoS ONE 10(7): e0132054. distribution maps for 2010 and were projected forward using the IPCC (Intergovernmental doi:10.1371/journal.pone.0132054 Panel on Climate Change) A2 scenario to predict distributions of the same species for Editor: Franck Courchamp, Ecologie, Systématique 2100. We found that distributions of cold-climate, northern, and interior small mammal spe- & Evolution, FRANCE cies experienced large decreases in area while shifting northward, upward in elevation, and Received: January 16, 2015 inland across the state. In contrast, many southern and continental species expanded Accepted: June 9, 2015 throughout Alaska, and also moved down-slope and toward the coast. -
Quilt Bookmarks.Indd
Activities based on the book Nature’s Patchwork Quilt - by Mary Miche’ Creating Nature Play Introduction In the book Nature’s Patchwork Quilt, author Mary Miche introduces children to a variety of habitats, from the forest to the ocean, and the rainforest to the arctic. In this activity children create a three-di- mensional diorama of one of the habitats from the book. Materials Needed Key Concepts • A habitat is the specific environment in which • copies of black and white habitat pages, 1 per child plants and animals live. colored pens • Different plants and animals inhabit different • habitats and have external features that help them adhesive tape • thrive. modeling clay • • Animals eat plants or other animals for food and • cardboard shoe boxes, 1 per child may also use plants or even other animals for • scissors shelter and nesting. • the book Nature’s Patchwork Quilt • Plants and animals within a habitat depend on one another (interdependence). Procedure 1. Give each child one of the habitat pages making sure that all habitats are represented. Using the book as a reference, have children color their page. 2. Instruct children cut off one of the long sides of the shoebox. Be sure to keep the bottom of the shoebox intact. Have children tape their colored habitat page to the bottom of the shoebox to create a backdrop for their diorama. 3. Using the clay, have children make several animals that live in the habitat, showing how they interact with the plants and other animals. 4. Display the finished dioramas and have children observe and discuss how the animals are interacting in each of the different habitats. -

Wildlife of the Far North
© Copyright, Princeton University Press. No part of this book may be distributed, posted, or reproduced in any form by digital or mechanical means without prior written permission of the publisher. MAMMALS ALL MAMMALS, from humans to lemmings to muskoxen to whales, share certain physical features. All mammals possess modified sweat glands called mammary glands, which in a female can produce milk. All have body hair, at least at the beginning of their lives, and all have a four-chambered heart, single-boned lower jaw, and a middle ear composed of three bones. Arctic mammals are warm-blooded, or endo- To prevent excessive heat loss from bare or lengthy thermic, creatures. They are able to maintain body parts, pinnipeds, caribou, and beavers a constant body temperature despite changing maintain two internal temperatures—a high body climatic conditions. Their core body temperatures core temperature and a much cooler temperature range from 97.7°F to 105°F (36.5°C–40.5°C), this in the flippers, legs, or tail, respectively. This is despite the fact that marine species live in seawater known as regional heterothermy, which is made of 28°F (−2°C) and land mammals experience possible through heat exchangers that shunt winter temperatures averaging −33°F (−36°C). cooled blood to the extremities before returning Most of the northern mammals have it to be warmed in the countercurrent system. developed well-insulated, compact bodies Walruses have a similar heat-exchange with short appendages, which minimize heat mechanism that controls blood flow to the loss and conserve body heat. Mammals that skin capillaries.