Moose Management Areas in Labrador !
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-
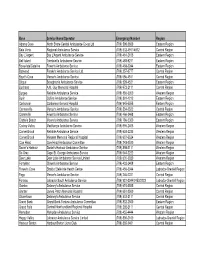
Revised Emergency Contact #S for Road Ambulance Operators
Base Service Name/Operator Emergency Number Region Adams Cove North Shore Central Ambulance Co-op Ltd (709) 598-2600 Eastern Region Baie Verte Regional Ambulance Service (709) 532-4911/4912 Central Region Bay L'Argent Bay L'Argent Ambulance Service (709) 461-2105 Eastern Region Bell Island Tremblett's Ambulance Service (709) 488-9211 Eastern Region Bonavista/Catalina Fewer's Ambulance Service (709) 468-2244 Eastern Region Botwood Freake's Ambulance Service Ltd. (709) 257-3777 Central Region Boyd's Cove Mercer's Ambulance Service (709) 656-4511 Central Region Brigus Broughton's Ambulance Service (709) 528-4521 Eastern Region Buchans A.M. Guy Memorial Hospital (709) 672-2111 Central Region Burgeo Reliable Ambulance Service (709) 886-3350 Western Region Burin Collins Ambulance Service (709) 891-1212 Eastern Region Carbonear Carbonear General Hospital (709) 945-5555 Eastern Region Carmanville Mercer's Ambulance Service (709) 534-2522 Central Region Clarenville Fewer's Ambulance Service (709) 466-3468 Eastern Region Clarke's Beach Moore's Ambulance Service (709) 786-5300 Eastern Region Codroy Valley MacKenzie Ambulance Service (709) 695-2405 Western Region Corner Brook Reliable Ambulance Service (709) 634-2235 Western Region Corner Brook Western Memorial Regional Hospital (709) 637-5524 Western Region Cow Head Cow Head Ambulance Committee (709) 243-2520 Western Region Daniel's Harbour Daniel's Harbour Ambulance Service (709) 898-2111 Western Region De Grau Cape St. George Ambulance Service (709) 644-2222 Western Region Deer Lake Deer Lake Ambulance -

Canada Canada De La
c FISHERIES AND MARINE SERVICE SERVICE DES P£CHES ET DES SCIENCES DE LA MER TECHNICAL REPORT No. 691 RAPPORT TECHNIQUE N° 1977 Environment Environnement I Canada Canada Fisheries Service des piches and Marine et des sciences Service de la mer Technical Reports Technical Reports are research documents that are of sufficient importance to be preserved, but which for some reason are not appropriate for primary scientific publication. Inquiries concerning any particular RepOlt should be directed to the issuing establishment. Rapports Techniques Les rapports techniques sont des documents de recherche qui revetent une assez grande importance pour etre conserves mais qui, pour une raison ou pour une autre, ne conviennent pas a une publication scientifique prioritaire. Pour toute demande de renseignements concernant un rapport particulier, i1 faut s'adresser au service responsable. Department of the Environment Ministere de 1'Environnement Fisheries and Marine Service Service des Peches et des Sciences de la mer Research and Development Directorate Direction du Recherche et Developpement • TECHNICAL REPORT No. 691 RAPPORT TECHNIQUE NO. 691 (Numbers 1-456 in this series (Les numeros 1-456 dans cette serie furent were issued as Technical Reports utilises comme Rapports Techniques de 1'office of the Fisheries Research Board of des recherches sur les p~cheries du Canada. Canada. The series name was changed Le nom de la serie fut change avec le with report number 457). rapport numero 457). • Limnology and Fish Populations of Red Indian Lake, a Multi-Use Reservoir by C.J. MORRY and L.J. COLE This is the forty-seventh Ceci est le quarante-septieme Technical Report from the Rapport Technique de la Direction du Research and Development Directorate Recherche et Developpement Newfoundland Biological Station Station biologique de Terre-Neuve St. -

Teaching & Learning Assistant Permanent Positions
Teaching & Learning Assistant Permanent Positions Amos Comenius Memorial School (Hopedale) J.R. Smallwood Memorial School (Wabush) Labrador Straits Academy (L’Anse Au Loup) Lake Melville School (North West River) Peacock Primary School (Happy Valley-Goose Bay) What is a TEACHING AND LEARNING ASSISTANT? The TLA is responsible for supporting teaching and learning in an inclusive environment in the areas of planning, instruction, social emotional learning, assessment, evaluation and collection of student data. Reporting to the Principal of the school the TLA will work with the classroom teacher to: ● Assist with the planning and organization of learning experiences for students in accordance with established policies and procedures. ● Assist the classroom teacher in implementing programs and services for students including the delivery of instructional supports. ● Assist the teacher in assessing, evaluating, reporting and recording student progress. ● Work collaboratively with the classroom teacher to develop supportive educational environments ● Participate as a member of school teams in meeting the needs of students. ● Maintain effective professional working relationships. Qualifications: Teacher’s Certificate Level Two (2) from the Registrar of Teacher Certification and Records at the Department of Education and Early Childhood Development. How to obtain this Level 2 certification: Application form Educational requirements: Completion of a minimum of 20 courses (i.e. 60 credit hours) from Memorial University (or another approved university). Courses completed in the areas of Education, Social Sciences or Psychology will be considered an asset OR; minimum Level 2 Early Childhood Educator certification from the Association of Early Childhood Educators of Newfoundland and Labrador. Salary: Certificate Level Two (2) of the Teachers Salary Grid; $37,476 - $45,794 per annum Once certified apply to the NLESD Website Personnel Package for Teaching and Learning Assistant Vacant Positions. -

Dukes County Intelligencer
Journal of History of Martha’s Vineyard and the Elizabeth Islands THE DUKES COUNTY INTELLIGENCER VOL. 55, NO. 1 WINTER 2013 Left Behind: George Cleveland, George Fred Tilton & the Last Whaler to Hudson Bay Lagoon Heights Remembrances The Big One: Hurricane of ’38 Membership Dues Student ..........................................$25 Individual .....................................$55 (Does not include spouse) Family............................................$75 Sustaining ...................................$125 Patron ..........................................$250 Benefactor...................................$500 President’s Circle ......................$1000 Memberships are tax deductible. For more information on membership levels and benefits, please visit www.mvmuseum.org To Our Readers his issue of the Dukes County Intelligencer is remarkable in its diver- Tsity. Our lead story comes from frequent contributor Chris Baer, who writes a swashbuckling narrative of two of the Vineyard’s most adventur- ous, daring — and quirky — characters, George Cleveland and George Fred Tilton, whose arctic legacies continue to this day. Our second story came about when Florence Obermann Cross suggested to a gathering of old friends that they write down their childhood memories of shared summers on the Lagoon. The result is a collective recollection of cottages without electricity or water; good neighbors; artistic and intellectual inspiration; sailing, swimming and long-gone open views. This is a slice of Oak Bluffs history beyond the more well-known Cottage City and Campground stories. Finally, the Museum’s chief curator, Bonnie Stacy, has reminded us that 75 years ago the ’38 hurricane, the mother of them all, was unannounced and deadly, even here on Martha’s Vineyard. — Susan Wilson, editor THE DUKES COUNTY INTELLIGENCER VOL. 55, NO. 1 © 2013 WINTER 2013 Left Behind: George Cleveland, George Fred Tilton and the Last Whaler to Hudson Bay by Chris Baer ...................................................................................... -

NEWFOUNDLAND and LABRADOR COLLEGE of OPTOMETRISTS Box 23085, Churchill Park, St
NEWFOUNDLAND AND LABRADOR COLLEGE OF OPTOMETRISTS Box 23085, Churchill Park, St. John's, NL A1B 4J9 Following are the names of Optometrists registered with the Newfoundland and Labrador College of Optometrists as of 1 January 2014 who hold a therapeutic drug certificate and may prescribe a limited number of medications as outlined in the following regulation: http://www.assembly.nl.ca/Legislation/sr/Regulations/rc120090.htm#3_ DR. ALPHONSUS A. BALLARD, GRAND FALLS-WINDSOR, NL DR. JONATHAN BENSE, ST. JOHN’S, NL DR. GARRY C. BEST, GANDER, NL DR. JUSTIN BOULAY, ST. JOHN’S, NL DR. LUC F. BOULAY, ST. JOHN'S, NL DR. RICHARD A. BUCHANAN, SPRINGDALE, NL DR. ALISON CAIGER-WATSON, GRAND FALLS-WINDSOR, NL DR. JOHN M. CASHIN, ST. JOHN’S, NL DR. GEORGE COLBOURNE, CORNER BROOK, NL DR. DOUGLAS COTE, PORT AUX BASQUES, NL DR. CECIL J. DUNCAN, GRAND FALLS-WINDSOR, NL DR. CARL DURAND, CORNER BROOK, NL DR. RACHEL GARDINER, GOULDS, NL DR. CLARE HALLERAN, CLARENVILLE, NL DR. DEAN P. HALLERAN, CLARENVILLE, NL DR. DEBORA HALLERAN, CLARENVILLE, NL DR. KEVIN HALLERAN, MOUNT PEARL, NL DR. ELSIE K. HARRIS, STEPHENVILLE, NL DR. JESSICA HEAD, GRAND FALLS-WINDSOR, NL 1 of 3 DR. IAN HENDERSON, ST. JOHN'S, NL DR. PAUL HISCOCK, ST. JOHN'S, NL DR. LISA HOUNSELL, ST. JOHN’S, NL DR. RICHARD J. HOWLETT, GRAND FALLS-WINDSOR, NL DR. SARAH HUTCHENS, ST. JOHN’S, NL DR. GRACE HWANG, GRAND FALLS-WINDSOR, NL DR. PATRICK KEAN, BAY ROBERTS, NL DR. NADINE KIELLEY, ST. JOHN’S, NL DR. CHRISTIE LAW, ST. JOHN’S, NL DR. ANGELA MacDONALD, SYDNEY, NS DR. -

Newfoundland in International Context 1758 – 1895
Newfoundland in International Context 1758 – 1895 An Economic History Reader Collected, Transcribed and Annotated by Christopher Willmore Victoria, British Columbia April 2020 Table of Contents WAYS OF LIFE AND WORK .................................................................................................................. 4 Fog and Foundering (1754) ............................................................................................................................ 4 Hostile Waters (1761) .................................................................................................................................... 4 Imports of Salt (1819) .................................................................................................................................... 5 The Great Fire of St. John’s (1846) ................................................................................................................. 5 Visiting Newfoundland’s Fisheries in 1849 (1849) .......................................................................................... 9 The Newfoundland Seal Hunt (1871) ........................................................................................................... 15 The Inuit Seal Hunt (1889) ........................................................................................................................... 19 The Truck, or Credit, System (1871) ............................................................................................................. 20 The Preparation of -

Lower Churchill Hydroelectric Generation Project: Community Health Study
Component Studies Socio-Economic Environment Socio-Economic Report 2 of 6 Community Health Study January 2009 Environmental Impact Statement for the Lower Churchill Hydroelectric Generation Project LOWER CHURCHILL HYDROELECTRIC GENERATION PROJECT: COMMUNITY HEALTH STUDY ENVIRONMENTAL BASELINE REPORT LCP 535785 FINAL REPORT DECEMBER 22, 2008 Aura Environmental Research and Consulting Ltd. for Minaskuat Inc. Lower Churchill Hydroelectric Generation Project ACKNOWLEDGEMENTS We wish to acknowledge Mark Shrimpton, Linda Jefferson and Colleen Leader with Minaskuat Inc. for providing study guidance and logistical support. We also acknowledge the following individuals for providing data and assistance with identifying key issues and health concerns pertinent to the communities and the region: • Doug Abbass (Principal, Peacock Primary School, Happy Valley-Goose Bay) • Dr. Maureen Baikie (Medical Officer of Health, Labrador Grenfell Health) • Andrew Battcock (Labrador School Board) • Carol Best (Labrador Central Economic Development Board) • Teresa Bruce (Retired Teacher, Sheshatshiu) • Delia Connell (Community and Aboriginal Affairs, Labrador-Grenfell) • Genevieve Corbin (Regional Director, Child Youth and Family Services) • Deanne Costello (Regional Director, Mental Health and Addictions Services) • Dolores Dooley (Program Consultant, Health and Community Services) • Blenda Dredge (Regional Director, Rehabilitation and Intervention Services) • Jan Dymond (Regional Director, Consumers’ Health Awareness Network Newfoundland and Labrador) • Nicole -

Eastern Labrador Field Excursion for Explorationists
EASTERN LABRADOR FIELD EXCURSION FOR EXPLORATIONISTS Charles F. Gower Geological Survey, Department of Natural Resources, Newfoundland and Labrador, P.O. Box 8700, St. John’s, Newfoundland, A1B 4J6. with contributions from James Haley and Chris Moran Search Minerals Inc., Suite 1320, 855 West Georgia St., Vancouver, B.C., V6C 3E8 and Alex Chafe Silver Spruce Resources Inc., Suite 312 – 197 Dufferin Street, Bridgewater, Nova Scotia, B4V 2G9. Open File LAB/1583 St. John’s, Newfoundland September, 2011 NOTE Open File reports and maps issued by the Geological Survey Division of the Newfoundland and Labrador Department of Natural Resources are made available for public use. They have not been formally edited or peer reviewed, and are based upon preliminary data and evaluation. The purchaser agrees not to provide a digital reproduction or copy of this product to a third party. Derivative products should acknowledge the source of the data. DISCLAIMER The Geological Survey, a division of the Department of Natural Resources (the “authors and publish- ers”), retains the sole right to the original data and information found in any product produced. The authors and publishers assume no legal liability or responsibility for any alterations, changes or misrep- resentations made by third parties with respect to these products or the original data. Furthermore, the Geological Survey assumes no liability with respect to digital reproductions or copies of original prod- ucts or for derivative products made by third parties. Please consult with the Geological Survey in order to ensure originality and correctness of data and/or products. Recommended citation: Gower, C.F., Haley, J., Moran, C. -

(PL-557) for NPA 879 to Overlay NPA
Number: PL- 557 Date: 20 January 2021 From: Canadian Numbering Administrator (CNA) Subject: NPA 879 to Overlay NPA 709 (Newfoundland & Labrador, Canada) Related Previous Planning Letters: PL-503, PL-514, PL-521 _____________________________________________________________________ This Planning Letter supersedes all previous Planning Letters related to NPA Relief Planning for NPA 709 (Newfoundland and Labrador, Canada). In Telecom Decision CRTC 2021-13, dated 18 January 2021, Indefinite deferral of relief for area code 709 in Newfoundland and Labrador, the Canadian Radio-television and Telecommunications Commission (CRTC) approved an NPA 709 Relief Planning Committee’s report which recommended the indefinite deferral of implementation of overlay area code 879 to provide relief to area code 709 until it re-enters the relief planning window. Accordingly, the relief date of 20 May 2022, which was identified in Planning Letter 521, has been postponed indefinitely. The relief method (Distributed Overlay) and new area code 879 will be implemented when relief is required. Background Information: In Telecom Decision CRTC 2017-35, dated 2 February 2017, the Canadian Radio-television and Telecommunications Commission (CRTC) directed that relief for Newfoundland and Labrador area code 709 be provided through a Distributed Overlay using new area code 879. The new area code 879 has been assigned by the North American Numbering Plan Administrator (NANPA) and will be implemented as a Distributed Overlay over the geographic area of the province of Newfoundland and Labrador currently served by the 709 area code. The area code 709 consists of 211 Exchange Areas serving the province of Newfoundland and Labrador which includes the major communities of Corner Brook, Gander, Grand Falls, Happy Valley – Goose Bay, Labrador City – Wabush, Marystown and St. -

Labrador City and Wabush : Resilient Communities Karen Oldford - Mayor Town of Labrador City Ken Anthony – CAO Town of Wabush
Labrador City and Wabush : Resilient Communities Karen Oldford - Mayor Town of Labrador City Ken Anthony – CAO Town of Wabush Benefits of Labrador West Established community = reduced start up costs for industry Suited for operation phase of projects Experience of labour force Resource companies share in the value of creating community through corporate stewardship. Community amenities key for retaining workers. Link to natural environment – recreation amenities. Boom Bust Cycle Last big Bust 1982 Hundreds of homes vacant for approx 8 yrs Homes sold by banks and companies for $5,000- $25,000 Affordable homes and affordable apartment rents until 2005 2010 – same homes sell for $325,000 - $549,000 rental rates now $1,000 per bedroom i.e. 1 bedroom apt 1,200, 2 bedroom $2,000 and house rental $5,000 month! Challenges of Labrador West • Economic dependence on single industry • Growth impaired due to subsurface mineral rights • Transient Workforce Population • Expense of building/operating in remote northern location Challenges: Single Industry Market is volatile Community grows and diminishes in response to the resource Non renewable = finite. Challenges: Growth • Expansion/development encroach on mineral reserves • Land management strongly influenced by Provincial interest and local industry • Growth responds to market conditions – often lags behind needs of community and industry Challenges: Flyin/Flyout Arrangements Necessary for resource projects when workforce needs are high but short lived. I.e. construction phase Transient residents – not fully engaged in community (i.e. lack of community involvement and volunteerism. Often project negative image of the region due to their lived reality.) Negative perception amongst long-term residents of “contractors”. -

Carol Inn Listing Flyer.Indd
HOTEL INVESTMENT PROPERTY CAROL INN 215 Drake Avenue, Labrador City, NL HOTEL ACQUISITION OPPORTUNITY CBRE, as the exclusive advisor to PwC, is pleased to present for sale the Carol Inn (the “Hotel” or “Property”). ROB COLEMAN +1 709 754 1454 Offi ce The Carol Inn is located within the Central Business District of Labrador City with high visibility via Drake Avenue. The hotel is +1 709 693 3868 Cell approximately 4.7 kilometers north of the Wabush Airport. [email protected] Strategically located within Lab West, the property features visibilty from the Trans-Labrador Highway, allowing for easy access to all high LLOYD NASH volume roads within the community. +1 709 754 0082 Offi ce +1 709 699 7508 Cell The Hotel has a total of 22 guest rooms, offering a mix of room types to meet leisure, corporate, and extended-stay demand. Signifi cant [email protected] rennovations have been undertaken in recent years to the hotel. The Property also contains basement meeting rooms, main level bar, 140 Water Street, Suite 705 dining room and restaurant. Oppotunities exist for redevelopment, of St. John`s, NL A1C 6H6 those areas currently not in operation. Fax +1 709 754 1455 This property has abundant parking and the potential for expansion. This unique opportunity rarely comes to market in Lab West - a rare commercial building, strategically located in the heart of Lab West. HOTEL ACQUISITION OPPORTUNITY INVESTMENT PROFILE | CAROL INN INVESTMENT HIGHLIGHTS STRONG LOCATION The Carol Inn is located in Labrador City, on the mainland portion of the province of Newfoundland and Labrador. -

Student and Youth Services Agreement.Xlsx
2015-16 Student and Youth Services Agreement Approvals Organization Location Project Approved Amount BOTWOOD BOYS AND GIRLS CLUB Botwood Youth Cordinator$ 40,100 CBDC TRINITY CONCEPTION CORPORATION Carbonear Community Youth Coordinator$ 87,500 COLLEGE OF THE NORTH ATLANTIC St. John's Small Enterprise Co-Operative Placement Assistance$ 229,714 COLLEGE OF THE NORTH ATLANTIC St. John's Student Works and Service Program (SWASP)$ 90,155 COLLEGE OF THE NORTH ATLANTIC St. John's Partnership in Academic Career Education Employment Program $ 57,232 (PACEE) COMMUNITY YOUTH NETWORK CORNER BROOK & AREA Corner Brook Impact $ 5,071 CONSERVATION CORPS NEWFOUNDLAND AND St. John's Green Team$ 579,600 LABRADOR FOR THE LOVE OF LEARNING, INC St. John's Watering the Seeds$ 115,000 HARBOUR BRETON COMMUNITY YOUTH Harbour Breton Youth Entreprensurial Skills$ 35,000 HARBOUR BRETON COMMUNITY YOUTH Harbour Breton Youth Outreach Coordinator$ 52,500 HARBOUR GRACE COMMUNITY YOUTH Harbour Grace Changing Lanes$ 60,301 KANGIDLUASUK STUDENT PROGRAM INC Nain Student Program$ 7,000 MARINE INSTITUTE St. John's Youth Opportinities Coop Program$ 100,000 MARINE INSTITUTE St. John's Wage Subsidies for MESD$ 11,186 MEMORIAL UNIVERSITY OF NL St. John's Small Enterprise Co-Operative Placement Assistance$ 522,993 MEMORIAL UNIVERSITY OF NL St. John's Graduate Transition to Employment (GTEP)$ 200,000 MEMORIAL UNIVERSITY OF NL St. John's Student Works and Service Program (SWASP)$ 331,680 MEMORIAL UNIVERSITY OF NL St. John's Partnership in Academic Career Education Employment Program $ 67,732 (PACEE) NEWFOUNDLAND AND LABRADOR ASSOC OF Mount Pearl Youth Ventures$ 82,000 COMMUNITY BUSINESS DEVELOPMENT CORPORATIONS SKILLS CANADA-NEWFOUNDLAND AND LABRADOR St.