Contraception Update
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-
A History of Birth Control Methods
Report Published by the Katharine Dexter McCormick Library and the Education Division of Planned Parenthood Federation of America 434 West 33rd Street, New York, NY 10001 212-261-4716 www.plannedparenthood.org Current as of January 2012 A History of Birth Control Methods Contemporary studies show that, out of a list of eight somewhat effective — though not always safe or reasons for having sex, having a baby is the least practical (Riddle, 1992). frequent motivator for most people (Hill, 1997). This seems to have been true for all people at all times. Planned Parenthood is very proud of the historical Ever since the dawn of history, women and men role it continues to play in making safe and effective have wanted to be able to decide when and whether family planning available to women and men around to have a child. Contraceptives have been used in the world — from 1916, when Margaret Sanger one form or another for thousands of years opened the first birth control clinic in America; to throughout human history and even prehistory. In 1950, when Planned Parenthood underwrote the fact, family planning has always been widely initial search for a superlative oral contraceptive; to practiced, even in societies dominated by social, 1965, when Planned Parenthood of Connecticut won political, or religious codes that require people to “be the U.S. Supreme Court victory, Griswold v. fruitful and multiply” — from the era of Pericles in Connecticut (1965), that finally and completely rolled ancient Athens to that of Pope Benedict XVI, today back state and local laws that had outlawed the use (Blundell, 1995; Himes, 1963; Pomeroy, 1975; Wills, of contraception by married couples; to today, when 2000). -

Contraception and Abortion Contraception Contraception Methods of Contraception Coitus Interruptus (Withdrawal)
12/3/2013 Contraception Contraception and • The intentional prevention of pregnancy Abortion – Birth control- the device or practice to decrease risk of conceiving – Family planning – conscious decision on when to conceive • More than half of pregnancies each year are unintended in Foundations of MCH women under 20 years • Risk of pregnancy with contraception – Using an imperfect method of contraception – Using a method of contraception incorrectly • Decreasing the risk of pregnancy – Providing adequate instruction about how to use contraceptive methods, when to use a backup method, & when to use emergency contraception Contraception Methods of Contraception • Contraceptive failure • Coitus Interruptus – The percentage of contraceptive users expected to have • Natural Family Planning & Fertility Awareness an accidental pregnancy during the first year, even Methods when use of methods is consistent and correct Barrier Methods – Effectiveness varies from couple to couple • • Properties of the method • Hormonal Methods • Characteristics of the user • Emergency Contraception • Failure rates decrease over time • Intrauterine Devices – Users gain experience • Sterilization – Users use methods more appropriately • Future Trends – Less effective users stop using the methods Natural Family Planning & Fertility Coitus Interruptus (Withdrawal) Awareness Methods • Involves the male partner withdrawing the • Knowledge of the menstrual cycle is basic to the practice of NFP penis from the woman’s vagina before he – Human ovum can be fertilized no later -

U.S. Medical Eligibility Criteria for Contraceptive Use, 2016
Morbidity and Mortality Weekly Report Recommendations and Reports / Vol. 65 / No. 3 July 29, 2016 U.S. Medical Eligibility Criteria for Contraceptive Use, 2016 U.S. Department of Health and Human Services Centers for Disease Control and Prevention Recommendations and Reports CONTENTS Introduction ............................................................................................................1 Methods ....................................................................................................................2 How to Use This Document ...............................................................................3 Keeping Guidance Up to Date ..........................................................................5 References ................................................................................................................8 Abbreviations and Acronyms ............................................................................9 Appendix A: Summary of Changes from U.S. Medical Eligibility Criteria for Contraceptive Use, 2010 ...........................................................................10 Appendix B: Classifications for Intrauterine Devices ............................. 18 Appendix C: Classifications for Progestin-Only Contraceptives ........ 35 Appendix D: Classifications for Combined Hormonal Contraceptives .... 55 Appendix E: Classifications for Barrier Methods ..................................... 81 Appendix F: Classifications for Fertility Awareness–Based Methods ..... 88 Appendix G: Lactational -

An Introduction and High Yield Summary of Female Contraceptive
Florida International University FIU Digital Commons HWCOM Faculty Publications Herbert Wertheim College of Medicine 10-24-2017 An Introduction and High Yield Summary of Female Contraceptive Methods Dharam Persaud Herbert Wertheim College of Medicine, Florida International University, [email protected] J. Burns Herbert Wertheim College of Medicine, Florida International University J. Trangle Herbert Wertheim College of Medicine, Florida International University J. Agudelo Honors College, Florida International University JA Gonzalez Honors College, Florida International University See next page for additional authors This work is licensed under a Creative Commons Attribution 4.0 License. Follow this and additional works at: https://digitalcommons.fiu.edu/com_facpub Part of the Medicine and Health Sciences Commons Recommended Citation Persaud, Dharam; Burns, J.; Trangle, J.; Agudelo, J.; Gonzalez, JA; Nunez, D.; Perez, K.; Rasch, D.; Valencia, S.; and Rao, C. V., "An Introduction and High Yield Summary of Female Contraceptive Methods" (2017). HWCOM Faculty Publications. 117. https://digitalcommons.fiu.edu/com_facpub/117 This work is brought to you for free and open access by the Herbert Wertheim College of Medicine at FIU Digital Commons. It has been accepted for inclusion in HWCOM Faculty Publications by an authorized administrator of FIU Digital Commons. For more information, please contact [email protected]. Authors Dharam Persaud, J. Burns, J. Trangle, J. Agudelo, JA Gonzalez, D. Nunez, K. Perez, D. Rasch, S. Valencia, and C. V. Rao This article is available at FIU Digital Commons: https://digitalcommons.fiu.edu/com_facpub/117 Open Access Austin Journal of Reproductive Medicine & Infertility Research Article An Introduction and High Yield Summary of Female Contraceptive Methods Persaud-Sharma D1*, Burns J1, Trangle J1, Agudelo J2, Gonzalez JA2, Nunez D2, Perez K2, Abstract Rasch D2, Valencia S2 and Rao CV1,3 Globally, contraceptive studies and their use are major challenges in the 1Florida International University, Herbert Wertheim realm of public health. -

A Study of the Vaginal Contraceptive Sponge Used with and Wtthout the Fertility Awareness Method
CONTACETIO A STUDY OF THE VAGINAL CONTRACEPTIVE SPONGE USED WITH AND WTTHOUT THE FERTILITY AWARENESS METHOD 1 Barbara Kass-Annese, RNCNP2 Kathy Irene Kennedy, MA MD3 Katherine Forrest, 4 Hal Danzer, MD 5 Anthony Reading, PhD6 Holly Hughes, MS 1Los Angeles Regional Family Planning Council, Los 2Family Angeles,CA Health International, Durham, NC 3Menlo Park, CA 4Fertility Institute, Beverly Hills, CA 5Cedar Sinai Medical 6 . Center, Los Angeles, CA Louisiana State University, Baton Rouge, LA ABSTRACT The actual effectiveness rates of natural and barrier methods of family planning are lower than the theoretical ones. If couples accurately defined the limits of the fertile phase and used barriers at that time, then actual effectiveness might increase. A randomized, controlled clinical trial was initiated to determine the effectiveness of the contraceptive sponge used only during the fertile time and to compare this with sponge use at every intercourse. Recruitment problems and discontinuation forced the early termination of this study, but qualitative information about compliance and dcceptability was collected. Common sponge problems were reported as were misuses of the sponge, but problems and misuse were not related. Determination of the fertile phase -as reportedly easy, but complaints of and discontinuation for inconvenience occurred. For unplanned pregnancies, contraceptive behaviors around the time of conception are presented. Submitted for publication August 14, 1989 Accepted for publication September 7, 1989 DECEMBER 1989 VOL. 40 NO. 6 701 CONTRACEPTION INTRODUCTION The actual effectiveness rates of all temporary contraceptives are generally not as great as their theoretical rates would predict. For barrier methods, the difference is presumably due to either the failure to use the method according to instruction or the failure to use them at every intercourse. -

Contraceptive Sponge
Contraceptive Sponge What is it? A soft, disposable, non-latex foam (polyurethane) device that is placed in the vagina before sex. The sponge contains a spermicide, which blocks or kills sperm. How does it work? • Fits over the cervix (entrance to uterus) • Traps, absorbs, and weakens the sperm (male reproductive cells) • Works for up to 12 hours Advantages • Does not contain hormones • Can be used by women who are breastfeeding • Can be used by women who smoke • One size fits all women • Does not require a prescription • Can be used for multiple acts of sex within a 12 hour time period Considerations • Must be available at the time of sex • Must be left in place for six hours following sex but no longer than 12 hours • Must be put in correctly • May irritate the vagina, which may increase the risk for contracting human immunodeficiency virus (HIV) • Cannot be used by people who are allergic to spermicides • If left in the vagina longer than the recommended time, symptoms of toxic shock syndrome may occur (fever, shock, problems with body organs) How to use the sponge Insert into the vagina at least 15 minutes prior to sex. Make sure to follow package instructions. Typical success rate Successful for 6.8 to 8.4 people out of 10. Sexually transmitted infection (STI) protection The contraceptive sponge does not protect against sexually transmitted infections (STIs). Use a latex condom, dental dam, or glove every time you have sex. References: The Society of Obstetricians and Gynecologists. (2009). Choosing a contraceptive that is right for u. -

Birth Control Methods
F REQUENTLY ASKED QUESTIONS Q: What are the different types of birth control? Birth Control A: You can choose from many methods of birth control. They are grouped by how Methods they work: Types of Birth Control Q: What is the best method of Continuous Abstinence birth control (or contraception)? Natural Family Planning/ http://www.womenshealth.gov A: There is no “best” method of birth control. Each method has its pros and Rhythm Method 1-800-994-9662 cons. Barrier Methods TDD: 1-888-220-5446 All women and men can have control • Contraceptive Sponge over when, and if, they become parents. • Diaphragm, Cervical Cap, and Making choices about birth control, Cervical Shield or contraception, isn’t easy. There are many things to think about. To get • Female Condom started, learn about birth control meth- • Male Condom ods you or your partner can use to pre- Hormonal Methods vent pregnancy. You can also talk with • Oral Contraceptives — Combined your doctor about the choices. pill (“The pill”) Before choosing a birth control meth- • Oral Contraceptives — Progestin- od, think about: only pill (“Mini-pill”) • Your overall health • The Patch • How often you have sex • Shot/Injection • The number of sex partners you • Vaginal Ring have Implantable Devices • If you want to have children some- • Implantable Rods day • Intrauterine Devices • How well each method works to prevent pregnancy Permanent Birth Control Methods • Possible side effects • Sterilization Implant • Your comfort level with using the method • Surgical Sterilization Keep in mind, even the most effective Emergency Contraception birth control methods can fail. But your chances of getting pregnant are lowest if the method you choose always is used correctly and every time you have sex. -

2017 Formulary Drug List
2017 Formulary Drug List For Small Groups and Large Groups GlobalHealth, Inc. 701 NE 10th Street, Suite 300 Oklahoma City, OK 73104-5403 MGDF17 Lists Updated 12/2017 www.GlobalHealth.com/commercial HELPFUL NUMBERS Plan Issuer: Dental: Mail Claims to: GlobalHealth, Inc. Careington BenefitSolutions Express Scripts PO Box 2393 1.866.636.9188 (toll-free) Attn: Commercial Claims Oklahoma City, OK 73101- www.careington.com/co/glob PO Box 14711 2393 alhealth Lexington, KY 40512-4711 GlobalHealth Customer Mail Claims to: Mail Order Pharmacy: Care, Language Assistance, Careington BenefitSolutions Express Scripts Customer and Disease Management: Claims Processing Center Service Center CommercialAnswers@global PO Box 60 1.866.274.1612 (toll-free) health.com Frisco, TX 75043 1.800.899.2114 (TTY) 405.280.2964 (local) 24 hours/7 days a week 1.877.280.2964 (toll-free) Dental Network: www.express-scripts.com 711 (TTY) www.careington.com/co/globalhealt Monday – Friday, 9 am – 5 h *Specialty Pharmacy: pm Central Accredo Specialty Pharmacy www.globalhealth.com/comm Pharmacy Benefits 1.888.608.9010 ercial Manager: www.accredo.com Express Scripts Holding Behavioral Health and Company 24/7 Nurse Help Line: Substance Use: 1.866.274.1612 (toll-free) Information Line CommercialAnswers@global 1.800.899.2114 (TTY) 1.877.280.2993 (toll-free) health.com 405.280.2964 (local) Medication Prior GlobalHealth Compliance 1.877.280.2964 (toll-free) Authorizations: Officer: 711 (TTY) [email protected] 1.877.280.5852 (toll-free) Monday – Friday, 9 am – 5 om 405.280.5852 pm Central 918.878.7361 [email protected] www.globalhealth.com/comm m ercial GlobalHealth Privacy Officer: 405.280.5524 [email protected] Spanish (Español): Para obtener asistencia en Español llame al 1-877-280-2964. -

Nonhormonal Contraception.Qxd
AMERICAN SOCIETY FOR REPRODUCTIVE MEDICINE 1209 Montgomery Highway • Birmingham, Alabama 35216-2809 • TEL (205) 978-5000 • FAX (205) 978-5005 • E-MAIL [email protected] • URL www.asrm.org PATIENT FACT SHEET Nonhormonal Contraception Despite a large number of contraceptive options available to couples, it is Another commonly practiced method of natural family planning is with- still thought that 50% of pregnancies in the United States are unintended. drawal, in which the man does not ejaculate inside the vagina at the time of Therefore, it is important to choose a contraceptive that can be used male orgasm. However, this method has a high rate of failure. consistently and correctly. The use of natural family planning can fail 2% to 25% of the time, When deciding on a contraceptive method, you need to think about how depending on the experience of the couple practicing this method. Women effective and convenient the contraceptive method is, how long it lasts, with irregular periods, abnormal bleeding, or cervical or vaginal infections whether it can be reversed, its side effects, price, and if it provides cannot use natural family planning because they cannot predict when they protection against sexually transmitted infections. may ovulate. Certain medications and medical conditions can change a woman's cervical mucus or body temperature, so these women should not How does contraception work? use natural family planning. Contraceptive agents work in many ways to prevent a pregnancy. They are usually divided into those that are either hormonal or nonhormonal. Most Intrauterine devices of the hormonal contraceptives work by changing a woman's hormone Intrauterine devices (IUDs) are safe and effective forms of long-term levels to mimic a pregnancy, therefore preventing eggs from being able to reversible contraception. -
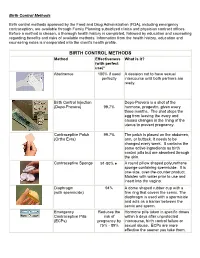
Birth Control Methods
Birth Control Methods Birth control methods approved by the Food and Drug Administration (FDA), including emergency contraception, are available through Family Planning subsidized clinics and physician contract offices. Before a method is chosen, a thorough health history is completed, followed by education and counseling regarding benefits and risks of available methods. Information from the health history, education and counseling notes is incorporated into the client's health profile. BIRTH CONTROL METHODS Method Effectiveness What is it? (with perfect use)* Abstinence 100% if used A decision not to have sexual perfectly intercourse until both partners are ready. Birth Control Injection Depo-Provera is a shot of the (Depo-Provera) 99.7% hormone, progestin, given every three months. The shot stops the egg from leaving the ovary and causes changes in the lining of the uterus to prevent pregnancy. Contraceptive Patch 99.7% The patch is placed on the abdomen, (Ortho Evra) arm, or buttock. It needs to be changed every week. It contains the same active ingredients as birth control pills but are absorbed through the skin. Contraceptive Sponge 91-80% ♦ A round pillow shaped polyurethane sponge containing spermicide. It is one-size, over-the-counter product. Moisten with water prior to use and insert into the vagina. Diaphragm 94% A dome shaped rubber cup with a (with spermicide) firm ring that covers the cervix. The diaphragm is used with a spermicide and acts as a barrier between the cervix and sperm. Emergency Reduces the Hormone pills taken in specific doses Contraceptive Pills risk of within 5 days after unprotected (ECPs) pregnancy by intercourse, birth control failure or 75% - 89% sexual abuse. -

Contraceptive Evidence Questions and Answers Second Edition
CONTRACEPTIVE EVIDENCE QUESTIONS AND ANSWERS SECOND EDITION www.prb.org POPULATION REFERENCE BUREAU ACKNOWLEDGMENTS The original edition of this publication was written by Mia Foreman, formerly of Population Reference Bureau (PRB) and Jeff Spieler, formerly of U.S Agency for International Development (USAID). The 2020 edition was updated with the help of a team of people at PRB, including Jerry Parks, Barbara Seligman, Heidi Worley, Paola Scommegna, Lillian Kilduff, Charlotte Greenbaum, Debbie Mesce, Liz Leahy Madsen, and Kaitlyn Patierno. PRB is grateful to the following reviewers for their time and insights: Shelley Snyder, Clive Mutunga, Kelly Thomas, Tabitha Sripipatana, Kevin Peine, and Abdulmumin Saad at USAID; and Laneta Dorflinger, Amanda Troxler, Kavita Nanda, Markus Steiner, and Elena Lebetkin at FHI360. This publication is made possible by the generous support of USAID under cooperative agreement AID-AA-A-16-00002. The information provided in this document is the responsibility of PRB, is not official U.S. government information, and does not necessarily reflect the views or positions of USAID or the U.S. Government. © 2020 Population Reference Bureau. All rights reserved. POPULATION REFERENCE BUREAU The Population Reference Bureau INFORMS people around the world about population, health, and the environment, and EMPOWERS them to use that information to ADVANCE the well-being of current and future generations. www.prb.org POPULATION REFERENCE BUREAU 1875 Connecticut Ave., NW 202 483 1100 PHONE Suite 520 202 328 3937 FAX Washington, -

Natural Family Planning ………………………………………………………
1 Summer/Fall 2012 ● Vol. 23, Nos. 3 & 4 Richard J. Fehring, PhD, RN, FAAN – Professor, Marquette University College of Nursing In this issue Natural Family Planning ……………………………………………………….. 2 Fertility …………………………………………………………………………... 3 Infertility ………………………………………………….……………………... 5 Abortion …...…………………………………………………………………….. 9 Pregnancy ……………………………………………………………………..... 12 Under the Microscope ………...……………………………………………...... 13 Fertility Awareness in Gynecology: Application to Family Planning and Endometriosis Current Medical Research is a publication of the Natural Family Planning Program of the United States Conference of Catholic Bishops.© Washington, DC: USCCB, 2012. The managing editor is Theresa Notare, PhD, Assistant Director. Permission is granted to reproduce in whole or in part, in print and/or electronically, with the following statement: Current Medical Research, NFPP/US Conference of Catholic Bishops, Washington, DC, vol# (year): page#. Used with permission. 2 Natural Family Planning Knowledge of Effectiveness of Natural Family Planning and Contraceptive Methods Found to Be Poor Central among the many issues that form a person’s choice in family planning is the method effectiveness rate for pregnancy avoidance (typically, most people do not consider the difference between moral and immoral methods). Despite the fact that couples who have just reasons to avoid pregnancy want a secure method of family planning, they often base their decisions on false perceptions of efficacy per method. Due to this tendency, researchers at Washington University conducted a study to determine the knowledge of contraceptive effectiveness among a cohort of women from Saint Louis.1 The women were enrolled in a project call Contraceptive CHOICE. The project had a larger, more global purpose—to promote the use of long acting reversible contraceptives (LARC), e.g., hormonal implants and intrauterine devices.