Neuro-Urology for the Urogynaecologist and Urologist W42, 30 August 2011 14:00 - 18:00
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

A New Look at the Etiology of Interstitial Cystitis/Bladder Pain Syndrome: Extraordinary Cultivations
International Urology and Nephrology (2019) 51:1961–1967 https://doi.org/10.1007/s11255-019-02248-5 UROLOGY - ORIGINAL PAPER A new look at the etiology of interstitial cystitis/bladder pain syndrome: extraordinary cultivations Tahsin Batuhan Aydogan1 · Oznur Gurpinar2 · Ozgen Koseoglu Eser2 · Begum Aydogan Mathyk3 · Ali Ergen1 Received: 25 April 2019 / Accepted: 24 July 2019 / Published online: 30 July 2019 © Springer Nature B.V. 2019 Abstract Purpose So far, studies have not clearly identifed infectious agents as an etiological factor for interstitial cystitis (IC). Spe- cifc microbiological diagnosis for detecting the pathogen with higher sensitivity in IC may decrease the treatment costs and increase psychosocial health of the patients. Methods A prospective clinical study was performed in 26 IC patients and 20 controls between April and September 2017. All participants were asked to give mid-stream urine sample for routine urine cultures. Followed by the negative results, symptomatic 26 patients were evaluated for L-form pathogen existence by extraordinary cultivation methods. Biopsy sam- ples were taken from 19 patients with ulcerative lesions in the bladder while collecting sterile urine samples from all 26 patients. PG broth, 5% sheep blood agar, EMB, Sabouraud’s dextrose, LEM, and GYPA were used. Followed by the 1st day inoculations, all inoculated PG broths were subcultured into the same solid media at the 2nd and 10th days in case of any growth after incubation of 24 h under 35–37 °C. The “O’Leary Sant Symptom and Problem Index” score forms were used to evaluate response to the appropriate treatment for those patients with documented pathogens. -

Interstitial Cystitis/Painful Bladder Syndrome
What I need to know about Interstitial Cystitis/Painful Bladder Syndrome U.S. Department of Health and Human Services National Kidney and Urologic Diseases NATIONAL INSTITUTES OF HEALTH Information Clearinghouse What I need to know about Interstitial Cystitis/Painful Bladder Syndrome U.S. Department of Health and Human Services National Kidney and Urologic Diseases NATIONAL INSTITUTES OF HEALTH Information Clearinghouse Contents What is interstitial cystitis/painful bladder syndrome (IC/PBS)? ............................................... 1 What are the signs of a bladder problem? ............ 2 What causes bladder problems? ............................ 3 Who gets IC/PBS? ................................................... 4 What tests will my doctor use for diagnosis of IC/PBS? ............................................................... 5 What treatments can help IC/PBS? ....................... 7 Points to Remember ............................................. 14 Hope through Research........................................ 15 Pronunciation Guide ............................................. 16 For More Information .......................................... 17 Acknowledgments ................................................. 18 What is interstitial cystitis/painful bladder syndrome (IC/PBS)? Interstitial cystitis*/painful bladder syndrome (IC/PBS) is one of several conditions that causes bladder pain and a need to urinate frequently and urgently. Some doctors have started using the term bladder pain syndrome (BPS) to describe this condition. Your bladder is a balloon-shaped organ where your body holds urine. When you have a bladder problem, you may notice certain signs or symptoms. *See page 16 for tips on how to say the words in bold type. 1 What are the signs of a bladder problem? Signs of bladder problems include ● Urgency. The feeling that you need to go right now! Urgency is normal if you haven’t been near a bathroom for a few hours or if you have been drinking a lot of fluids. -
Symptoms of Overactive Bladder and Interstitial Cystitis – How Different?
183 Homma Y1, Tanaka M1, Niimi A1 1. Japan Red Cross Medical Centre SYMPTOMS OF OVERACTIVE BLADDER AND INTERSTITIAL CYSTITIS – HOW DIFFERENT? Hypothesis / aims of study Overactive bladder (OAB) is a symptom syndrome of urgency, frequency or urgency incontinence [1]. Interstitial cystitis (IC) is a bladder disease with a symptom syndrome of urgency, frequency, or bladder pain. Difference in symptoms of these confusable disorders is to be examined. Study design, materials and methods Female patients with OAB or IC and asymptomatic controls were recruited for the study. Diagnosis of OAB is based on 1) OAB symptom score [2] larger than 5 and/or urgency incontinence, and 2) exclusion of stress urinary incontinence and other obvious diseases. IC was diagnosed by 1) O’Leary & Sant’s symptom score [3] larger than 7 and/or bladder pain, 2) exclusion of other obvious diseases, and 3) Hunner’s ulcer or bladder bleeding at hydrodistension under anesthesia. The subjects recorded, in 3-day bladder diary, time voided, voided volume and intensity (0: none, 1: slightly, 2: moderately, 3: a lot) of 6 sensations or symptoms (urge to void, fear of leakage, amount of leakage, bladder discomfort, bladder pain and feeling of incomplete emptying) for each micturition. Additionally the urge questionnaire of 5 questions addressing the conditions during the last week was administered: 1) Did you feel urge to void that was not followed by voiding? (Response: yes, no), 2) Did you feel strong urge to void? (yes, no), 3) In case of yes in question 2, did you feel strong urge to void suddenly? (yes, no), 4) What happened or might happen, if you held strong urge to void for a long time, 1 hour for example ? (Response: leak urine, feel discomfort and/or pain, both), 5) In case of discomfort and/or pain in question 4, could you tell these 2 sensations apart? (yes, no). -
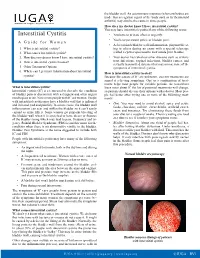
Interstitial Cystitis? You May Have Interstitial Cystitis If Any of the Following Occur: Interstitial Cystitis • You Have to Urinate Often Or Urgently
the bladder wall. An autoimmune response (when antibodies are made that act against a part of the body such as in rheumatoid arthritis) may also be the cause in some people. How does my doctor know I have interstitial cystitis? You may have interstitial cystitis if any of the following occur: Interstitial Cystitis • You have to urinate often or urgently. • You have persistent pelvic or bladder pain. A Guide for Women • A doctor finds bladder wall inflammation, pinpoint bleed- 1. What is interstitial cystitis? ing or ulcers during an exam with a special telescope 2. What causes interstitial cystitis? (called a cystoscope) used to look inside your bladder. 3. How does my doctor know I have interstitial cystitis? • Your doctor has ruled out other diseases such as urinary 4. How is interstitial cystitis treated? tract infections, vaginal infections, bladder cancer, and sexually transmitted diseases that may mimic some of the 5. Other Treatment Options symptoms of interstitial cystitis. 6. Where can I get more information about interstitial How is interstitial cystitis treated? cystitis? Because the causes of IC are unknown, current treatments are aimed at relieving symptoms. One or a combination of treat- ments helps most people for variable periods. As researchers What is interstitial cystitis? learn more about IC the list of potential treatments will change, Interstitial cystitis (IC) is a term used to describe the condition so patients should discuss their options with a doctor. Most peo- of bladder pain or discomfort with a frequent and often urgent ple feel better after trying one or more of the following treat- need to pass urine. -

Associated Diseases in Interstitial Cystitis/Bladder Pain Syndrome
Associated diseases in interstitial cystitis/bladder pain syndrome Joop P. van de Merwe [email protected] Associated diseases in IC/BPS patients Table 1. Examples of associated disorders diagnosed in IC/BPS patients in comparison with Associated diseases are diseases that occur more often the general population 2,5,6 together in the same person than by chance. Associated diseases for IC/BPS (interstitial cystitis/bladder pain diagnosis prevalence (%) syndrome) include allergy, fibromyalgia, irritable bowel IC/BPS general syndrome, Crohn's disease, ulcerative colitis, systemic lupus population erythematosus, rheumatoid arthritis and Sjögren's syndrome allergy 40.6 22.5 (table 1). irritable bowel syndrome 25.4 2.9 sensitive skin 22.6 10.6 Allergy vulvodynia 10.9 15.0 An allergy occurs when a person's immune system causes an fibromyalgia 12.8 3.2 adverse reaction to substances in the environment that are chronic fatigue syndrome 7.7 8.5 harmless to most other people. These substances (so-called migraine 18.8 18.0 allergens) are found in dust mites, pets, pollen, insects, ticks, asthma 9.2 6.1 15 moulds, foods and many medications. Adverse reactions Crohn’s disease/ulcerative colitis 7.3 0.07 without involvement of the immune system are called rheumatoid arthritis 4-13 1.0 intolerance. Intolerance mainly occurs to food ingredients and systemic lupus erythematosus 1.7 0.05 drugs. Sjögren’s syndrome 8.0 0.5 People experience different symptoms, depending on the allergen, the individual's immune system, previous contact with the same allergen and where it enters the body. -

Interstitial Cystitis
DIAGNOSIS AND TREATMENT OF INTERSTITIAL CYSTITIS Women with interstitial cystitis (IC) often feel the need to urinate frequently WHAT IS INTERSTITIAL in addition to experiencing painful urination even though bladder infection CYSTITIS (PAINFUL is not the cause. For many women IC interrupts their normal daily activities BLADDER SYNDROME)? because of the need to stay close to a bathroom and a constant feeling of discomfort. Interstitial cystitis (IC), also DIAGNOSING INTERSTITIAL CYSTITIS known as painful bladder Chesapeake Urology’s pelvic health specialists will run a series of diagnostic syndrome, is a chronic tests for other conditions that cause the same symptoms as IC and come to infl ammatory condition a diagnosis once all other causes are ruled out. Our experienced physicians of the bladder lining that perform a comprehensive physical exam and may order additional tests including: causes pain and pressure • Urine analysis and urine culture in the pelvic area around • Cystoscopy with bladder distention the bladder. • Biopsy of the bladder • CT scan of the bladder IC affects approximately • Urodynamic testing eight million young and middle-aged women in the HOW IS IC TREATED? U.S. While there is no cure for IC, treatments can provide relief from painful symptoms. Our physicians provide several different therapies that have Symptoms of IC can been shown to alleviate and/or diminish many of the symptoms of IC include: including: • Pain in the bladder • Physical therapy – As a treatment for underlying pelvic fl oor dysfunction, physical -

Underlying Causes of Chronic Bladder Dysfunction
South African Family Practice 2018; 60(2):24-27 S Afr Fam Pract Open Access article distributed under the terms of the ISSN 2078-6190 EISSN 2078-6204 Creative Commons License [CC BY-NC-ND 4.0] © 2018 The Author(s) http://creativecommons.org/licenses/by-nc-nd/4.0 REVIEW Underlying causes of chronic bladder dysfunction Andre Marais,1 Senior lecturer and Clinical Pharmacologist, Elzbieta Osuch,2 Professor and Clinical Pharmacologist 1Department of Pharmacology, School of Medicine, Faculty of Health Sciences, University of Pretoria, South Africa 2Department of Pharmacology& Therapeutics, School of Medicine, Sefako Makghato Health Sciences University, South Africa Corresponding author, email: [email protected] / [email protected] Abstract General practitioners and family physicians frequently encounter patients suffering from voiding disorders. Various underlying causes such as spinal cord injury, cerebrovascular accidents, traumatic brain injury and several neurological diseases including Parkinson’s disease, dementia, multiple sclerosis and vitamin deficiencies have been implicated. Chronic bladder conditions may cause social embarrassment and decrease quality of life in affected individuals. It is important to identify the most common underlying causes of bladder dysfunction and have adequate knowledge regarding the primary treatment and prevention of complications in order to reduce the economic burden created by this group of disorders. Keywords: Chronic bladder conditions; interstitial cystitis; painful bladder syndrome; chronic -

Interstitial Cystitis (IC) Is a Chronic Inflammatory Condition of the Bladder Wall Which Frequently Goes Undiagnosed
Interstitial Cystitis (IC) is a chronic inflammatory condition of the bladder wall which frequently goes undiagnosed. Although the exact cause is unknown and no treatment works for everyone, many treatments are available and most patients get relief. "Common" cystitis, also known as a urinary tract infection (UTI), is caused by bacteria and is usually successfully treated with antibiotics. Unlike common cystitis, IC is not caused by bacteria, therefore it won't respond to antibiotics. For many women it takes years to get the correct diagnosis so they are made to feel like it is all in their head...it's not! Symptoms FREQUENCY: Day and/or night frequency of urination. In early or mild cases, frequency may be the only symptom. URGENCY: The sensation of having to urinate immediately, which may also be accompanied by pain, pressure or spasms. PAIN: Can be in the lower abdomen, urethra or vaginal area. Pain is frequently associated with sex. OTHER DISORDERS: Some IC patients report muscle and joint pain, migraines, allergies and GI problems. IC seems to have an association with other chronic diseases & pain syndromes such as vulvar vestibulitis, endometriosis, fibromyalgia & irritable bowel syndrome. Diagnosis IC is frequently misdiagnosed as UTI, which is easily treated with antibiotics. There isn't a single test that diagnoses IC, it is a diagnosis of exclusion, which means that if IC is suspected, the following tests may be performed to rule out other causes or further confirm the diagnosis of IC. 1. A urinalysis and urine culture: Other conditions need to be ruled out that have symptoms resembling IC. -

Urinary Bladder, Renal Pelvis & Urethra John F
Urinary Tract Pathology: Urinary Bladder, Renal Pelvis & Urethra John F. Madden, M.D., Ph.D. Spring 2010 Cystitis Infectious cystitis •“Ascending” infection due to enteric bacteria • >95% of cases due to E. coli • Klebsiella, Proteus, etc. in predisposed pts • Yeast, viruses (CMV, polyoma, adenovirus) with immunosuppression •Favored by obstruction •Prostatism, congenital anomalies, stones Urethral colonization Asymptomati c bacteriuria (<104/ml) “Urethral syndrome” (104–105/ml) Cystitis (≥105/ml) Pyelonephritis Interstitial (“Hunner’s”) cystitis • Idiopathic (? autoimmune, mast cell dysfunction) cystitis • Typically, women in later adulthood • Hematuria, pain • Extensive ulceration, often transmural, with fibrosis • dDx: infection, cancer Hemorrhagic cystitis • Complication of chemo-therapy or therapeutic pelvic irradiation • Cyclophosphamide, others • Can cause severe hemorrhage Malakoplakia & Xanthogranulomatous pyelonephritis •Chronic bacterial infection with ineffective clearance of organisms • Proteus often involved •“Pseudotumor” •Sheets of histiocytes packed lysosomes •Malakoplakia has Michaelis-Gutmann bodies Urothelial metaplasia • Urothelium takes on characteristics of some other type of epithelium • Often a response to chronic inflammation • Benign Normal urothelium Cystitis cystica Normal submucosal nests of urothelium (“von Brunn’s nests”) develop central cystic change Cystitis glandularis Transitional cells convert to mucinous columnar type Squamous metaplasia Transitional cells convert to squamous cells under chronic irritation -

Bladder Control, Bowel Control, Pelvic Pain and Sexual Dysfunction in Women
THE 4 UNMENTIONABLES: Bladder Control, Bowel Control, Pelvic Pain and Sexual Dysfunction in Women What every woman should know about causes, symptoms, diagnosis and treatment of four topics no one wants to talk about www.beaumont.org The 4 Unmentionables: Bladder Control, Bowel Control, Pelvic Pain and Sexual Dysfunction in Women THE 4 Beaumont’s Women’s Urology and Pelvic UNMENTIONABLES Health Center brings care for all of these services – bladder control, non-menstrual Bladder control, bowel control, pelvic pain, pelvic pain, bowel issues, and sexual and sexual dysfunction are four topics that dysfunction – under one roof. A nurse many women are uncomfortable talking about practitioner coordinates care between the because the symptoms can be embarrassing. Beaumont team and the woman’s primary Not only are these topics difficult to talk about, care physician. but many women accept them as a normal part of being female. Women tend to attribute incontinence, pelvic pain, and sexual problems as a normal part of having children or aging. “It’s not a normal part of the aging process,” says Kenneth Peters, M.D., chief of urology at NIH Beaumont Hospital, Royal Oak. 25% Dr. Peters points out that while these problems may impact many women, that doesn’t make them normal, and they certainly are treatable. • 25-45 percent of women have some form of urinary incontinence in their lifetime (NIH) 7-20% • 7-20 percent of the population suffer from constipation or fecal incontinence • 1 in 9 women suffer from pelvic pain • 3 million women in the U.S. may have interstitial cystitis (ICA) • 43 percent of women experience sexual dysfunction (JAMA) 3,000,000 Health care professionals have a responsibility have ICA to make sure women feel comfortable talking about these issues, so they can properly diagnose and treat their conditions, Dr. -

Urethral Syndrome in Women
STEVEN L. JENSEN, M.D. 200 S. Wenona Suite 298 Bay City, Michigan 48706 (989) 895-2634 (989) 895-2636 (Fax) Urethral Syndrome in Women Urethral syndrome describes a situation in which women suffer from a variety of irritative bladder symptoms that include more frequent urination, urgency (a stronger than normal urge to urinate), burning with urination, slowing of the stream, pain in the lower abdomen, a sense of incomplete emptying of the bladder after urinating, pain with intercourse and incontinence of urine (unwanted loss of urine). Not all women have all of the symptoms listed above. Definitions The urethra (your-e-thra) is a channel or tube through which urine flows from the bladder to the outside. In women, this channel ends just above the vaginal opening. The urethra is surrounded by muscles, which squeeze the channel shut, and gives us our urinary control. Cystitis (sis-tie-tus) is the medical term for lower urinary tract or bladder infection. Diagnosis Urethral syndrome is most often misdiagnosed as bladder infection of 'cystitis'. A bladder infection has identical symptoms but requires the presence of large amounts of bacteria in the urine to make a diagnosis. With cystitis, antibiotics are used and most symptoms resolve quickly after treatment. Patients with urethral syndrome have no bacteria in their urine and hence, do not respond to antibiotic therapy. An internal telescopic examination of the bladder (cystoscopy) in patients with bladder infection shows the wall to be red and inflamed. In patients with urethral syndrome is relatively rare, many women will be given multiple courses of different antibiotics just on the basis of symptoms. -

Pseudomembranous Cystitis: an Uncommon Ultrasound Appearance of Cystitis in Cats and Dogs
veterinary sciences Article Pseudomembranous Cystitis: An Uncommon Ultrasound Appearance of Cystitis in Cats and Dogs Caterina Puccinelli , Ilaria Lippi, Tina Pelligra, Tommaso Mannucci, Francesca Perondi , Mirko Mattolini and Simonetta Citi * Department of Veterinary Sciences, University of Pisa, Via Livornese Lato Monte, 56121 Pisa, Italy; [email protected] (C.P.); [email protected] (I.L.); [email protected] (T.P.); [email protected] (T.M.); [email protected] (F.P.); [email protected] (M.M.) * Correspondence: [email protected] Abstract: In veterinary medicine, pseudomembranous cystitis (PC) is a rare condition described only in cats. The purposes of this retrospective study were to describe ultrasound features of PC in cats and dogs, predisposing factors, comorbidities and outcomes. Cats and dogs with an ultrasonographic diagnosis of PC were included in the study. The bladder ultrasound findings that were recorded were: pseudomembranes’ characteristics, abnormalities of the bladder’s wall and content and anomalies of the pericystic peritoneal space. Ten cats and four dogs met the inclusion criteria. Four pseudomembrane adhesion patterns were described. The presence of pseudomembrane acoustic shadowing was observed in the 60% of cats. A total of 80% of the cats included were presented for urethral obstruction (UO) and/or had at least one episode of UO in the previous 2 months. Thirteen patients out of fourteen received only medical therapy, and all of them survived. PC is a rare disorder in cats and dogs and there are some ultrasonographic differences between the two species, suggesting Citation: Puccinelli, C.; Lippi, I.; a greater severity of the pathology in cats.