Bladder Pain Syndrome International Consultation on Incontinence
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Mimickers of Urothelial Carcinoma and the Approach to Differential Diagnosis
Review Mimickers of Urothelial Carcinoma and the Approach to Differential Diagnosis Claudia Manini 1, Javier C. Angulo 2,3 and José I. López 4,* 1 Department of Pathology, San Giovanni Bosco Hospital, 10154 Turin, Italy; [email protected] 2 Clinical Department, Faculty of Medical Sciences, European University of Madrid, 28907 Getafe, Spain; [email protected] 3 Department of Urology, University Hospital of Getafe, 28905 Getafe, Spain 4 Department of Pathology, Cruces University Hospital, Biocruces-Bizkaia Health Research Institute, 48903 Barakaldo, Spain * Correspondence: [email protected]; Tel.: +34-94-600-6084 Received: 17 December 2020; Accepted: 18 February 2021; Published: 25 February 2021 Abstract: A broad spectrum of lesions, including hyperplastic, metaplastic, inflammatory, infectious, and reactive, may mimic cancer all along the urinary tract. This narrative collects most of them from a clinical and pathologic perspective, offering urologists and general pathologists their most salient definitory features. Together with classical, well-known, entities such as urothelial papillomas (conventional (UP) and inverted (IUP)), nephrogenic adenoma (NA), polypoid cystitis (PC), fibroepithelial polyp (FP), prostatic-type polyp (PP), verumontanum cyst (VC), xanthogranulomatous inflammation (XI), reactive changes secondary to BCG instillations (BCGitis), schistosomiasis (SC), keratinizing desquamative squamous metaplasia (KSM), post-radiation changes (PRC), vaginal-type metaplasia (VM), endocervicosis (EC)/endometriosis (EM) (müllerianosis), -

Surgical Treatment of Urinary Incontinence in Men
Committee 13 Surgical Treatment of Urinary Incontinence in Men Chairman S. HERSCHORN (Canada) Members H. BRUSCHINI (Brazil), C.COMITER (USA), P.G RISE (France), T. HANUS (Czech Republic), R. KIRSCHNER-HERMANNS (Germany) 1121 CONTENTS I. INTRODUCTION VIII. TRAUMATIC INJURIES OF THE URETHRA AND PELVIC FLOOR II. EVALUATION PRIOR TO SURGICAL THERAPY IX. CONTINUING PEDIATRIC III. INCONTINENCE AFTER RADICAL PROBLEMS INTO ADULTHOOD: THE PROSTATECTOMY FOR PROSTATE EXSTROPHY-EPISPADIAS COMPLEX CANCER X. DETRUSOR OVERACTIVITY AND IV. INCONTINENCE AFTER REDUCED BLADDER CAPACITY PROSTATECTOMY FOR BENIGN DISEASE XI. URETHROCUTANEOUS AND V. SURGERY FOR INCONTINENCE IN RECTOURETHRAL FISTULAE ELDERLY MEN VI. INCONTINENCE AFTER XII. THE ARTIFICIAL URINARY EXTERNAL BEAM RADIOTHERAPY SPHINCTER (AUS) ALONE AND IN COMBINATION WITH SURGERY FOR PROSTATE CANCER XIII. SUMMARY AND RECOMMENDATIONS VII. INCONTINENCE AFTER OTHER TREATMENT FOR PROSTATE CANCER REFERENCES 1122 Surgical Treatment of Urinary Incontinence in Men S. HERSCHORN, H. BRUSCHINI, C. COMITER, P. GRISE, T. HANUS, R. KIRSCHNER-HERMANNS high-intensity focused ultrasound, other pelvic I. INTRODUCTION operations and trauma is a particularly challenging problem because of tissue damage outside the lower Surgery for male incontinence is an important aspect urinary tract. The artificial sphincter implant is the of treatment with the changing demographics of society most widely used surgical procedure but complications and the continuing large numbers of men undergoing may be more likely than in other areas and other surgery and other treatments for prostate cancer. surgical approaches may be necessary. Unresolved problems from pediatric age and patients with Basic evaluation of the patient is similar to other areas refractory incontinence from overactive bladders may of incontinence and includes primarily a clinical demand a variety of complex reconstructive surgical approach with history, frequency-volume chart or procedures. -

A New Look at the Etiology of Interstitial Cystitis/Bladder Pain Syndrome: Extraordinary Cultivations
International Urology and Nephrology (2019) 51:1961–1967 https://doi.org/10.1007/s11255-019-02248-5 UROLOGY - ORIGINAL PAPER A new look at the etiology of interstitial cystitis/bladder pain syndrome: extraordinary cultivations Tahsin Batuhan Aydogan1 · Oznur Gurpinar2 · Ozgen Koseoglu Eser2 · Begum Aydogan Mathyk3 · Ali Ergen1 Received: 25 April 2019 / Accepted: 24 July 2019 / Published online: 30 July 2019 © Springer Nature B.V. 2019 Abstract Purpose So far, studies have not clearly identifed infectious agents as an etiological factor for interstitial cystitis (IC). Spe- cifc microbiological diagnosis for detecting the pathogen with higher sensitivity in IC may decrease the treatment costs and increase psychosocial health of the patients. Methods A prospective clinical study was performed in 26 IC patients and 20 controls between April and September 2017. All participants were asked to give mid-stream urine sample for routine urine cultures. Followed by the negative results, symptomatic 26 patients were evaluated for L-form pathogen existence by extraordinary cultivation methods. Biopsy sam- ples were taken from 19 patients with ulcerative lesions in the bladder while collecting sterile urine samples from all 26 patients. PG broth, 5% sheep blood agar, EMB, Sabouraud’s dextrose, LEM, and GYPA were used. Followed by the 1st day inoculations, all inoculated PG broths were subcultured into the same solid media at the 2nd and 10th days in case of any growth after incubation of 24 h under 35–37 °C. The “O’Leary Sant Symptom and Problem Index” score forms were used to evaluate response to the appropriate treatment for those patients with documented pathogens. -

Interstitial Cystitis/Painful Bladder Syndrome
What I need to know about Interstitial Cystitis/Painful Bladder Syndrome U.S. Department of Health and Human Services National Kidney and Urologic Diseases NATIONAL INSTITUTES OF HEALTH Information Clearinghouse What I need to know about Interstitial Cystitis/Painful Bladder Syndrome U.S. Department of Health and Human Services National Kidney and Urologic Diseases NATIONAL INSTITUTES OF HEALTH Information Clearinghouse Contents What is interstitial cystitis/painful bladder syndrome (IC/PBS)? ............................................... 1 What are the signs of a bladder problem? ............ 2 What causes bladder problems? ............................ 3 Who gets IC/PBS? ................................................... 4 What tests will my doctor use for diagnosis of IC/PBS? ............................................................... 5 What treatments can help IC/PBS? ....................... 7 Points to Remember ............................................. 14 Hope through Research........................................ 15 Pronunciation Guide ............................................. 16 For More Information .......................................... 17 Acknowledgments ................................................. 18 What is interstitial cystitis/painful bladder syndrome (IC/PBS)? Interstitial cystitis*/painful bladder syndrome (IC/PBS) is one of several conditions that causes bladder pain and a need to urinate frequently and urgently. Some doctors have started using the term bladder pain syndrome (BPS) to describe this condition. Your bladder is a balloon-shaped organ where your body holds urine. When you have a bladder problem, you may notice certain signs or symptoms. *See page 16 for tips on how to say the words in bold type. 1 What are the signs of a bladder problem? Signs of bladder problems include ● Urgency. The feeling that you need to go right now! Urgency is normal if you haven’t been near a bathroom for a few hours or if you have been drinking a lot of fluids. -

Pediatric Hemorrhagic Cystitis
CUA BEST PRACTICE REPORT Canadian Urological Association Best Practice Report: Pediatric hemorrhagic cystitis Jessica H. Hannick, MD, MSc1,2; Martin A. Koyle, MD, MSc2,3 1Division of Pediatric Urology, UH Rainbow Babies and Children’s Hospital, Cleveland, OH, United States; 2The Hospital for Sick Children, Toronto, ON, Canada; 3Division of Urology, Department of Surgery, University of Toronto, Toronto, ON, Canada Cite as: Can Urol Assoc J 2019;13(11):E325-34. http://dx.doi.org/10.5489/cuaj.5993 tem for guideline recommendations, as employed by the International Consultation on Urologic Disease (ICUD). Published online March 29, 2019 Definition HC is defined by the presence of hematuria and lower uri- Introduction nary tract symptoms, such as dysuria, frequency, or urgen- cy, in the absence of other potential contributing factors, This best practice report aims to provide the general practic- such as vaginal bleeding or bacterial or fungal urinary tract ing urologist with basic background information regarding infections.1 Multiple grading criteria have been published the pathophysiology and natural history of hemorrhagic cys- to distinguish the varied presentations of HC. Frequently titis (HC) in the pediatric population, as well as diagnostic referenced grading schema are Droller and Arthur’s, which and algorithmic therapeutic recommendations. Given that are used to aid the clinician in discerning potential treatment HC in the pediatric population is most frequently diagnosed options and inform the clinician about prognosis (Table 1).2,3 in the setting of bone marrow (BMT) or stem cell transplan- The European Organization for Research and Treatment of tation (SCT), discussion and recommendations will focus Cancer has combined similar grading criteria, along with largely on this population, but many of the diagnostic and quality of life parameters to further relay the morbidity and treatment options can be expanded to broader pediatric mortality implications of each grade.4 populations affected by HC. -
Symptoms of Overactive Bladder and Interstitial Cystitis – How Different?
183 Homma Y1, Tanaka M1, Niimi A1 1. Japan Red Cross Medical Centre SYMPTOMS OF OVERACTIVE BLADDER AND INTERSTITIAL CYSTITIS – HOW DIFFERENT? Hypothesis / aims of study Overactive bladder (OAB) is a symptom syndrome of urgency, frequency or urgency incontinence [1]. Interstitial cystitis (IC) is a bladder disease with a symptom syndrome of urgency, frequency, or bladder pain. Difference in symptoms of these confusable disorders is to be examined. Study design, materials and methods Female patients with OAB or IC and asymptomatic controls were recruited for the study. Diagnosis of OAB is based on 1) OAB symptom score [2] larger than 5 and/or urgency incontinence, and 2) exclusion of stress urinary incontinence and other obvious diseases. IC was diagnosed by 1) O’Leary & Sant’s symptom score [3] larger than 7 and/or bladder pain, 2) exclusion of other obvious diseases, and 3) Hunner’s ulcer or bladder bleeding at hydrodistension under anesthesia. The subjects recorded, in 3-day bladder diary, time voided, voided volume and intensity (0: none, 1: slightly, 2: moderately, 3: a lot) of 6 sensations or symptoms (urge to void, fear of leakage, amount of leakage, bladder discomfort, bladder pain and feeling of incomplete emptying) for each micturition. Additionally the urge questionnaire of 5 questions addressing the conditions during the last week was administered: 1) Did you feel urge to void that was not followed by voiding? (Response: yes, no), 2) Did you feel strong urge to void? (yes, no), 3) In case of yes in question 2, did you feel strong urge to void suddenly? (yes, no), 4) What happened or might happen, if you held strong urge to void for a long time, 1 hour for example ? (Response: leak urine, feel discomfort and/or pain, both), 5) In case of discomfort and/or pain in question 4, could you tell these 2 sensations apart? (yes, no). -

149 Treatment with Instillation of Hyaluronic
149 Collado Serra A1, Lopez-Guerrero J A2, Dominguez-Escrig J2, Ramirez Backhaus M2, Gomez-Ferrez A2, Mir C3, Casanova J3, Iborra I3, Casaña M3, Solsona E3, Arribas L3, Rubio-Briones J3 1. Fundación IVO. Valencia.Spain, 2. Fundación IVO, Valencia, Spain, 3. Fundación IVO, Valencia,Spain. TREATMENT WITH INSTILLATION OF HYALURONIC ACID IN PATIENTS WITH A HISTORY OF PELVIC RADIATION THERAPY AND URINARY STORAGE SYMPTOMS Hypothesis / aims of study Pelvic radiotherapy for the treatment of tumours located in the pelvis is not free of the risk of secondary irradiation of the bladder, producing a certain histological changes and it can result in both acute and chronic bladder injuries. Of these, the most evident and studied is radiation-induced hemorrhagic cystitis, although other urinary symptoms like frequency, urgency, incontinence, dysuria or pelvic pain has been described. Therefore, the objective of our paper is to evaluate the clinical utility of bladder instillation of hyaluronic acid in patients with a history of pelvic radiation therapy and storage symptoms with failure of previous treatment with anticholinergic Study design, materials and methods We considered 39 consecutive patients with storage urinary symptoms (defined as urinary urgency, usually accompanied by frequency and nocturia, with or without urgency urinary incontinence) due to post radiation cystitis treated with bladder instillation of hyaluronic acid. Severe urgency episodes with or without incontinence was measured using the PPIUS (Patient Perception of Intensity of Urgency Scale). Eligible patients had ≥three severe urgency episodes with or without incontinence during the 3-day voiding diary period, defined as PPIUS grades 3 and 4, and ≥eight micturitions/24 hours (1). -
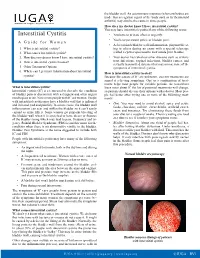
Interstitial Cystitis? You May Have Interstitial Cystitis If Any of the Following Occur: Interstitial Cystitis • You Have to Urinate Often Or Urgently
the bladder wall. An autoimmune response (when antibodies are made that act against a part of the body such as in rheumatoid arthritis) may also be the cause in some people. How does my doctor know I have interstitial cystitis? You may have interstitial cystitis if any of the following occur: Interstitial Cystitis • You have to urinate often or urgently. • You have persistent pelvic or bladder pain. A Guide for Women • A doctor finds bladder wall inflammation, pinpoint bleed- 1. What is interstitial cystitis? ing or ulcers during an exam with a special telescope 2. What causes interstitial cystitis? (called a cystoscope) used to look inside your bladder. 3. How does my doctor know I have interstitial cystitis? • Your doctor has ruled out other diseases such as urinary 4. How is interstitial cystitis treated? tract infections, vaginal infections, bladder cancer, and sexually transmitted diseases that may mimic some of the 5. Other Treatment Options symptoms of interstitial cystitis. 6. Where can I get more information about interstitial How is interstitial cystitis treated? cystitis? Because the causes of IC are unknown, current treatments are aimed at relieving symptoms. One or a combination of treat- ments helps most people for variable periods. As researchers What is interstitial cystitis? learn more about IC the list of potential treatments will change, Interstitial cystitis (IC) is a term used to describe the condition so patients should discuss their options with a doctor. Most peo- of bladder pain or discomfort with a frequent and often urgent ple feel better after trying one or more of the following treat- need to pass urine. -

Hemorrhagic Cystitis with Giant Cells in Rheumatoid Arthritis Treating with Tacrolimus
Journal of Rheumatic Diseases Vol. 21, No. 6, December, 2014 □ Case Report □ http://dx.doi.org/10.4078/jrd.2014.21.6.336 Hemorrhagic Cystitis with Giant Cells in Rheumatoid Arthritis Treating with Tacrolimus In Suk Min, YeonMi Ju, Hyun-young Kim, Yun Jung Choi, Won-Seok Lee, Wan-Hee Yoo Department of Rheumatology, Chonbuk National University Medical School and Research Institute of Clinical Medicine of Chonbuk National University Hospital-Chonbuk National University, Jeonju, Korea Hemorrhagic cystitis is a diffuse inflammation of the mu- hemorrhagic cystitis due to tacrolimus for the treatment cosa of the bladder, characterized by hematuria and burn- of rheumatoid arthritis. We describe a case of hemor- ing upon urination. This might be caused by a variety of rhagic cystitis with giant cells in a patient with rheumatoid reasons, including undergoing chemotherapy (such as cy- arthritis treating with tacrolimus. Hematuria resolved clophosphamide), radiation therapy, bladder cancer, cer- spontaneously with discontinuation of the drug. tain viruses, urinary infections, and thrombocytopenia. Key Words. Hemorrhagic cystitis, Rheumatoid arthritis, There are no previous reports of hemorrhagic cystitis asso- Tacrolimus ciated with the use of tacrolimus. This is the first case of Introduction with an occasional small clot. She was initially treated, em- Hemorrhagic cystitis (HC) is a diffuse inflammation of the pirically, with sulfamethoxazole/trimethoprim for a presumed mucosa of the bladder. It is characterized by gross hematuria urinary tract infection, but showed no change in symptoms. and irritating voiding symptoms such as dysuria, with fre- She was referred for further urologic evaluation. The patient quency and urgency (1). The reasons behind this may include denied any prior urologic history, and did not have a history undergoing chemotherapy (such as cyclophosphamide), using of smoking. -

Native Kidney Cytomegalovirus Nephritis and Cytomegalovirus Prostatitis in a Kidney Transplant Recipient
Received: 23 July 2018 | Revised: 20 August 2018 | Accepted: 2 September 2018 DOI: 10.1111/tid.12998 CASE REPORT Native kidney cytomegalovirus nephritis and cytomegalovirus prostatitis in a kidney transplant recipient Susanna K. Tan1 | Xingxing S. Cheng2 | Chia‐Sui Kao3 | Jenna Weber3 | Benjamin A. Pinsky1,3 | Harcharan S. Gill4 | Stephan Busque5 | Aruna K. Subramanian1 | Jane C. Tan2 1Department of Medicine, Division of Infectious Diseases and Geographic Abstract Medicine, Stanford University School of We present a case of cytomegalovirus (CMV) native kidney nephritis and prostatitis Medicine, Stanford, California in a CMV D+/R‐ kidney transplant recipient who had completed six months of CMV 2Department of Medicine, Division of Nephrology, Stanford University School of prophylaxis four weeks prior to the diagnosis of genitourinary CMV disease. The Medicine, Stanford, California patient had a history of benign prostatic hypertrophy and urinary retention that re‐ 3Department of Pathology, Stanford quired self‐catheterization to relieve high post‐voiding residual volumes. At 7 months University School of Medicine, Stanford, California post‐transplant, he was found to have a urinary tract infection, moderate hydrone‐ 4Department of Urology, Stanford University phrosis of the transplanted kidney, and severe hydroureteronephrosis of the native School of Medicine, Stanford, California left kidney and ureter, and underwent native left nephrectomy and transurethral re‐ 5Department of Surgery, Division of Abdominal Transplantation, Stanford section of the prostate. Histopathologic examination of kidney and prostate tissue University School of Medicine, Stanford, revealed CMV inclusions consistent with invasive CMV disease. This case highlights California that CMV may extend beyond the kidney allograft to involve other parts of the geni‐ Correspondence tourinary tract, including the native kidneys and prostate. -

EAU Guidelines on Bladder Stones 2019
EAU Guidelines on Bladder Stones C. Türk (Chair), A. Skolarikos (Vice-chair), J.F. Donaldson, A. Neisius, A. Petrik, C. Seitz, K. Thomas Guidelines Associate: Y. Ruhayel © European Association of Urology 2019 TABLE OF CONTENTS PAGE 1. INTRODUCTION 3 1.1 Aims and Scope 3 1.2 Panel Composition 3 1.3 Available Publications 3 1.4 Publication History and Summary of Changes 3 1.4.1 Publication History 3 2. METHODS 3 2.1 Data Identification 3 2.2 Review 4 3. GUIDELINES 4 3.1 Prevalence, aetiology and risk factors 4 3.2 Diagnostic evaluation 4 3.2.1 Diagnostic investigations 5 3.3 Disease Management 5 3.3.1 Conservative treatment and Indications for active stone removal 5 3.3.2 Medical management of bladder stones 5 3.3.3 Bladder stone interventions 5 3.3.3.1 Suprapubic cystolithotomy 5 3.3.3.2 Transurethral cystolithotripsy 5 3.3.3.2.1 Transurethral cystolithotripsy in adults: 5 3.3.3.2.2 Transurethral cystolithotripsy in children: 6 3.3.3.3 Percutaneous cystolithotripsy 6 3.3.3.3.1 Percutaneous cystolithotripsy in adults: 6 3.3.3.3.2 Percutaneous cystolithotripsy in children: 6 3.3.3.4 Extracorporeal shock wave lithotripsy (SWL) 6 3.3.3.4.1 SWL in Adults 6 3.3.3.4.2 SWL in Children 6 3.3.4 Treatment for bladder stones secondary to bladder outlet obstruction (BOO) in adult men 7 3.3.5 Urinary tract reconstructions and special situations 7 3.3.5.1 Neurogenic bladder 7 3.3.5.2 Bladder augmentation 7 3.3.5.3 Urinary diversions 7 4. -

(Part 1): Management of Male Urethral Stricture Disease
EURURO-9412; No. of Pages 11 E U R O P E A N U R O L O G Y X X X ( 2 0 2 1 ) X X X – X X X ava ilable at www.sciencedirect.com journa l homepage: www.europeanurology.com Review – Reconstructive Urology European Association of Urology Guidelines on Urethral Stricture Disease (Part 1): Management of Male Urethral Stricture Disease a, b c d Nicolaas Lumen *, Felix Campos-Juanatey , Tamsin Greenwell , Francisco E. Martins , e f a c g Nadir I. Osman , Silke Riechardt , Marjan Waterloos , Rachel Barratt , Garson Chan , h i a j Francesco Esperto , Achilles Ploumidis , Wesley Verla , Konstantinos Dimitropoulos a b Division of Urology, Gent University Hospital, Gent, Belgium; Urology Department, Marques de Valdecilla University Hospital, Santander, Spain; c d Department of Urology, University College London Hospital, London, UK; Department of Urology, Santa Maria University Hospital, University of Lisbon, e f Lisbon, Portugal; Department of Urology, Sheffield Teaching Hospitals, Sheffield, UK; Department of Urology, University Medical Center Hamburg- g h Eppendorf, Hamburg, Germany; Division of Urology, University of Saskatchewan, Saskatoon, Canada; Department of Urology, Campus Biomedico i j University of Rome, Rome, Italy; Department of Urology, Athens Medical Centre, Athens, Greece; Aberdeen Royal Infirmary, Aberdeen, UK Article info Abstract Article history: Objective: To present a summary of the 2021 version of the European Association of Urology (EAU) guidelines on management of male urethral stricture disease. Accepted May 15, 2021 Evidence acquisition: The panel performed a literature review on these topics covering a time frame between 2008 and 2018, and used predefined inclusion and exclusion criteria Associate Editor: for the literature to be selected.