Pilot Longitudinal Study on Post-Stroke Subjects
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Somatotopic Organization of Perioral Musculature Innervation Within the Pig Facial Motor Nucleus
Original Paper Brain Behav Evol 2005;66:22–34 Received: September 20, 2004 Returned for revision: November 10, 2004 DOI: 10.1159/000085045 Accepted after revision: December 7, 2004 Published online: April 8, 2005 Somatotopic Organization of Perioral Musculature Innervation within the Pig Facial Motor Nucleus Christopher D. Marshall a Ron H. Hsu b Susan W. Herring c aTexas A&M University at Galveston, Galveston, Tex., bDepartment of Pediatric Dentistry, University of North Carolina, Chapel Hill, N.C., and cDepartment of Orthodontics, University of Washington, Seattle, Wash., USA Key Words pools of the lateral 4 of the 7 subnuclei of the facial motor Somatotopy W Innervation W Facial nucleus W Perioral nucleus. The motor neuron pools of the perioral muscles muscles W Orbicularis oris W Buccinator W Mammals were generally segregated from motoneurons innervat- ing other facial muscles of the rostrum. However, motor neuron pools were not confined to single nuclei but Abstract instead spanned across 3–4 subnuclei. Perioral muscle The orbicularis oris and buccinator muscles of mammals motor neuron pools overlapped but were organized so- form an important subset of the facial musculature, the matotopically. Motor neuron pools of portions of the perioral muscles. In many taxa, these muscles form a SOO overlapped greatly with each other but exhibited a robust muscular hydrostat capable of highly manipula- crude somatotopy within the SOO motor neuron pool. tive fine motor movements, likely accompanied by a spe- The large and somatotopically organized SOO motor cialized pattern of innervation. We conducted a retro- neuron pool in pigs suggests that the upper lip might be grade nerve-tracing study of cranial nerve (CN) VII in pigs more richly innervated than the other perioral muscles (Sus scrofa) to: (1) map the motor neuron pool distribu- and functionally divided. -

A Systematic Review of Structural and Functional MRI Stud
Yin et al. The Journal of Headache and Pain (2020) 21:78 The Journal of Headache https://doi.org/10.1186/s10194-020-01131-4 and Pain REVIEW ARTICLE Open Access The neuro-pathophysiology of temporomandibular disorders-related pain: a systematic review of structural and functional MRI studies Yuanyuan Yin1,2†, Shushu He2†, Jingchen Xu2, Wanfang You1,3, Qian Li1,3, Jingyi Long1,3, Lekai Luo1,3, Graham J. Kemp4, John A. Sweeney1,5, Fei Li1,3*, Song Chen2* and Qiyong Gong1,3 Abstract Chronic pain surrounding the temporomandibular joints and masticatory muscles is often the primary chief complaint of patients with temporomandibular disorders (TMD) seeking treatment. Yet, the neuro-pathophysiological basis underlying it remains to be clarified. Neuroimaging techniques have provided a deeper understanding of what happens to brain structure and function in TMD patients with chronic pain. Therefore, we performed a systematic review of magnetic resonance imaging (MRI) studies investigating structural and functional brain alterations in TMD patients to further unravel the neurobiological underpinnings of TMD-related pain. Online databases (PubMed, EMBASE, and Web of Science) were searched up to August 3, 2019, as complemented by a hand search in reference lists. A total of 622 papers were initially identified after duplicates removed and 25 studies met inclusion criteria for this review. Notably, the variations of MRI techniques used and study design among included studies preclude a meta-analysis and we discussed the findings qualitatively according to the specific neural system or network the brain regions were involved in. Brain changes were found in pathways responsible for abnormal pain perception, including the classic trigemino-thalamo-cortical system and the lateral and medial pain systems. -

MR Imaging of Wallerian Degeneration in the Brainstem: Temporal Relationships
897 MR Imaging of Wallerian Degeneration in the Brainstem: Temporal Relationships Yuichi Inoue 1 Degeneration of the myelin sheath and axon distal to the most proximal site of axonal Yasumasa Matsumura2 interruption secondary to axonal disease has been called wallerian degeneration. On Teruo Fukuda1 MR imaging, wallerian degeneration of the pyramidal tract can be observed as an Yutaka Nemoto1 abnormal signal intensity, showing prolonged T1 and T2 relaxation times that correspond to the corticospinal tract, with or without shrinkage of the ipsilateral cerebral peduncle Nobuyuki Shirahata3 and pons. Review of MR studies in 150 cases of supratentorial cerebrovascular accidents Tosihisa Suzuki4 1 showed abnormal signal alterations in the ipsilateral brainstem in 33 of the cases. Miyuki Shakudo Abnormal intensity in the ipsilateral brainstem was seen as early as 5 weeks after the 1 Satoshi Yawata supratentorial ictus and was fully evident after 10 weeks in all 33 cases. Signal 2 Shigeko Tanaka alterations were strongest at about 3-6 months when compared with alterations seen Kazumasa Takemoto5 at 10 weeks or even 10 months after the ictus. Shrinkage of the ipsilateral brainstem Yasuto Onoyama 1 appeared as early as 8 months and was demonstrated in all cases 13 months after the ictus. MR seems to be the most effective technique for early detection of wallerian degen eration and may provide insight into its pathophysiological and chemical changes. AJNR 11:897-902, September/October 1990 Wallerian degeneration is the process of disintegration that affects an axon and its myelin sheath after its connection with the cell body has been interrupted [1 ). -

The Size and Distribution of Midbrain Dopaminergic Populations Are Permanently Altered by Perinatal Glucocorticoid Exposure in a Sex- Region- and Time-Specific Manner
Neuropsychopharmacology (2007) 32, 1462–1476 & 2007 Nature Publishing Group All rights reserved 0893-133X/07 $30.00 www.neuropsychopharmacology.org The Size and Distribution of Midbrain Dopaminergic Populations are Permanently Altered by Perinatal Glucocorticoid Exposure in a Sex- Region- and Time-Specific Manner Simon McArthur1, Emily McHale1 and Glenda E Gillies*,1 1 Department of Cellular and Molecular Neuroscience, Division of Neuroscience and Mental Health, Imperial College, London, UK Central dopaminergic (DA) systems appear to be particularly vulnerable to disruption by exposure to stressors in early life, but the underlying mechanisms are poorly understood. As endogenous glucocorticoids (GCs) are implicated in other aspects of neurobiological programming, this study aimed to characterize the effects of perinatal GC exposure on the cytoarchitecture of DA populations in the substantia nigra pars compacta (SNc) and the ventral tegmental area (VTA). Dexamethasone was administered non-invasively to rat pups via the mothers’ drinking water during embryonic days 16–19 or postnatal days 1–7, with a total oral intake circa 0.075 or 0.15 mg/ kg/day, respectively; controls received normal drinking water. Analysis of tyrosine hydroxylase-immunoreactive cell counts and regional volumes in adult offspring identified notable sex differences in the shape and volume of the SNc and VTA, as well as the topographical organization and size of the DA populations. Perinatal GC treatments increased the DA population size and altered the shape of the SNc and VTA as well as the organization of the DA neurons by expanding and/or shifting them in a caudal direction. This response was sexually dimorphic and included a feminization or demasculinization of the three-dimensional cytoarchitecture in males, and subtle differences that were dependent on the window of exposure. -
Lecture 6: Cranial Nerves
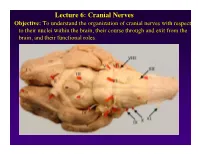
Lecture 6: Cranial Nerves Objective: To understand the organization of cranial nerves with respect to their nuclei within the brain, their course through and exit from the brain, and their functional roles. Olfactory Eye Muscles 3, 4 &6 Cranial Nerves 1-7 I overview Table, Page 49 II Lecture notes Cranial Nerves and their Functions V Trigeminal VII Facial VIII IX X XII XI Cranial Nerves 8-12 Overview sternocephalic I. Factors Responsible for the Complex Internal Organization of the Brain Stem-> leads to altered location of cranial nerve nuclei in adult brain stem 1. Development of the Fourth Ventricle a. Medulla and Pons develop ventral to the 4th ventricle cerebellum b. Alar plate is displaced lateral to basal plate 4 Medulla Developing Neural Tube 2. Cranial nerve nuclei form discontinuous columns Rostral 12 SE Page 48 Notes 3. Some cranial nerve nuclei migrate from their primitive embryonic positions (e.g., nuclei of V and VII) Facial N. Factors responsible for the complex internal organization of the brainstem: 4) Special senses develop in association with the brain stem. Nuclei of special senses 5) Development of the cerebellum and its connections Cerebellum II. Cranial Nerve Nuclei: Nucleus = column of neuron cell bodies. Efferent nuclei are composed of cell bodies of alpha or gamma motor neurons (SE) or preganglionic parasympathetic neurons (VE). III. Motor Efferent Nuclei (Basal Plate Derivatives): 1. SE (Somatic Efferent) Nuclei: SE neurons form two longitudinally oriented but discontinuous columns of cell bodies in the brain stem. Neurons that comprise these columns are responsible for innervating all of the skeletal musculature of the head. -

The High Yield Neurologic Examination Sadly, I Still Have Nothing New to Disclose Since Tuesday John Engstrom, M.D
Disclosures The High Yield Neurologic Examination Sadly, I still have nothing new to disclose since Tuesday John Engstrom, M.D. August 2018 Medical Chart Quotes Medical Chart Quotes “Exam of the genitalia reveals that he is circus sized.” “While in the ER, She was examined, X- rated and sent home.” 1 Overview – The Neurologic Medical Chart Quotes Examination • Mental status-brief review “Both breasts are equal and reactive • Cranial nerves – common/urgent patterns • Motor exam – common patterns to light and accommodation” • Sensory exam – common patterns • What is wrong with my walking? • Demonstrate the 15 minute exam • Other questions/demonstrations? Screening Mental Status Screening for Visual Field Deficits • Orientation-time, place, person • Visual field screen if you suspect a brain problem • Attention-Digit span forward (nl > 6-7) • Allows you to test function of broad areas of brain – Lobes-occipital, temporal, parietal • Language-repetition, naming, comprehen – Optic nerves, chiasm, optic tracts, and thalamus • Memory-Recall of 3 common objects at 5 • Clinical Importance minutes; if misses an answer give a prompt – “An anatomic sedimentation rate of the brain” • Abstractions-Similarities and differences – Detect abnormalities that require brain imaging (e.g.-apple vs. orange; lake vs. river) – Localize the deficit (right vs. left brain) 2 Screening for Visual Field Deficits- Ambulatory, Cooperative Patient • Imagine visual field cut in four equal pieces • Move examiner finger in the center of each quadrant with patient gaze fixed -

Trigeminal Neuropathy Accompanied by a Pontine Lesion on MRI
□ CASE REPORT □ Trigeminal Neuropathy Accompanied by a Pontine Lesion on MRI Yoichi Ohnuki, Atsushi Mizuma, Tsuyoshi Uesugi and Shunya Takizawa Abstract A 63-year-old man presented with the loss of the sensations of pain and temperature sensation in the right facial region innervated by the trigeminal nerve (V1 to 3). He showed abnormal lesions in the pons and the trigeminal nerve on magnetic resonance imaging (MRI). He had recurrent herpes in the nasal cavity, and a history of left facial palsy. We herein present the unique MRI findings and suggest that herpes simplex infec- tion may cause trigeminal neuropathy. This is the first reported case of dissociated trigeminal neuropathy with herpes simplex infection which was accompanied by a pontine lesion on MRI. Key words: dissociated trigeminal neuropathy, herpes simplex virus, MRI, pontine lesion (Intern Med 55: 1187-1189, 2016) (DOI: 10.2169/internalmedicine.55.5730) port of a patient with dissociated trigeminal sensory neuro- Introduction pathy, which was accompanied by a pontine lesion on MRI. The trigeminal nerve is the largest of the 12 cranial Case Report nerves. It carries the sensory input from the supratentorial dura mater, face, cornea, and the nasal, oral, and sinus mu- A 63-year-old man noticed right palatine numbness after cosa (1). The trigeminal nerve has three main branches dis- the appearance of herpes in his nasal cavity. It spread to his tal to the trigeminal ganglion: the ophthalmic (V1), maxil- right chin, temple and forehead over several days. He was lary (V2), and mandibular (V3) nerves, of which the latter admitted to our hospital on the 14th day after the onset of also carries efferent motor signals to the muscles of mastica- symptoms. -

How Is the Brain Organized?
p CHAPTER 2 How Is the Brain Organized? An Overview of Brain Structure The Functional Organization Brain Terminology of the Brain The Brain’s Surface Features Principle 1: The Sequence of Brain Processing The Brain’s Internal Features Is “In Integrate Out” Microscopic Inspection: Cells and Fibers Principle 2: Sensory and Motor Divisions Exist Focus on Disorders: Meningitis and Throughout the Nervous System Encephalitis Principle 3: The Brain’s Circuits Are Crossed Focus on Disorders: Stroke Principle 4: The Brain Is Both Symmetrical and Asymmetrical Principle 5: The Nervous System Works A Closer Look at Neuroanatomy Through Excitation and Inhibition The Cranial Nervous System Principle 6: The Central Nervous System Has The Spinal Nervous System Multiple Levels of Function The Internal Nervous System Principle 7: Brain Systems Are Organized Both Focus on Disorders: Magendie, Bell, and Bell’s Hierarchically and in Parallel Palsy Principle 8: Functions in the Brain Are Both Localized and Distributed A. Klehr / Stone Images Micrograph: Carolina Biological Supply Co. / Phototake 36 I p hen buying a new car, people first inspect the In many ways, examining a brain for the first time is outside carefully, admiring the flawless finish similar to looking under the hood of a car. We have a vague W and perhaps even kicking the tires. Then they sense of what the brain does but no sense of how the parts open the hood and examine the engine, the part of the car that we see accomplish these tasks. We may not even be responsible for most of its behavior—and misbehavior. able to identify many of the parts. -

Paraspinal Magnetic and Transcutaneous Electrical Stimulation
Encyclopedia of Computational Neuroscience DOI 10.1007/978-1-4614-7320-6_603-6 # Springer Science+Business Media New York 2014 Paraspinal Magnetic and Transcutaneous Electrical Stimulation Ursula S. Hofstoetter*, Simon M. Danner and Karen Minassian Center of Medical Physics and Biomedical Engineering, Medical University of Vienna, Institute of Analysis and Scientific Computing, Vienna University of Technology, Vienna, Austria Synonyms High-voltage paraspinal electrical stimulation; High-voltage percutaneous electrical stimulation; Magnetic paravertebral stimulation; Magnetic spinal stimulation; Paravertebral neuromagnetic stimulation; Spinal electromagnetic stimulation; Spinal neuromagnetic stimulation; Spinal root stimulation; Transcutaneous posterior root stimulation; Transcutaneous spinal cord stimulation; Transcutaneous spinal stimulation; Transspinal stimulation Definition Paraspinal magnetic and electrical stimulation target deep neural structures within the vertebral canal and in between neighboring vertebrae from a distance of several centimeters, with either magnetic coils or skin electrodes. The principal mechanism of stimulation at the neuronal level is the same for magnetic and electrical stimulation and is given by the induced electric field component or, in an equivalent way, the electric potential, generated along the anatomical path of the nerve fibers. Yet the generation of electric fields by electromagnetic induction or electrical stimulation bases on different physical principles, and the currents generated differ substantially, both as a function of time (pulse shape) and space (distribution in the body tissues). Induced ionic tissue currents secondary to externally applied, time-varying magnetic fields enter the spine predominantly from its sides, while the currents generated by transcutaneous electrical stimulation penetrate it mainly from its back (or front). The motor fibers within the spinal nerves and anterior rootlets are the common targets of paraspinal magnetic stimulation. -

Embryonic Development of the Leech Nervous System: Primary Axon Outgrowth of Identified Neurons’
0270-6474/83/0310-2098$02.00/O The Journal of Neuroscience Copyright 0 Society for Neuroscience Vol. 3, No. 10, pp. 2098-2111 Printed in U.S.A. October 1983 EMBRYONIC DEVELOPMENT OF THE LEECH NERVOUS SYSTEM: PRIMARY AXON OUTGROWTH OF IDENTIFIED NEURONS’ JOHN Y. KUWADA*y2 AND ANDREW P. KRAMER$ * Department of Biology, University of California, San Diego, La Jolla, California 92093 and $ Department of Molecular Biology, University of California, Berkeley, California 94720 Received January 21, 1983; Revised May 2, 1983; Accepted May 5, 1983 Abstract This paper describes the embryonic development of the leech nervous system and focuses on the differentiation of two identified pressure sensory (P) neurons, the Pn and Pv neurons. In the adult leech the P neurons have distinctive cell body locations in the central nervous system (CNS), different peripheral axon branching patterns, and different receptive field territories in the skin. The embryonic P neurons also have distinct and reproducible locations in the CNS and have been studied with recording and dye-filled microelectrodes from the time the first growth cones are projected from their somata. The peripheral axons of the P neurons are among the earliest peripheral axons to develop and may play an important role in the formation of peripheral nerves. The first or primary peripheral axons of the P neurons grow directly to their separate target territories. The specificity of the P neurons for their targets is probably not due to temporal differences in the outgrowth of their primary axons. Instead, the Pn neuron seems to exhibit a preference early in embryogenesis for the target of its primary axon despite an apparent opportunity to occupy the target of the Pv primary axon. -

Ch 7 the Nervous System Notes Lisa Peck
Name________________________________Block_____Date______________________ Ch 7 The Nervous System Notes Lisa Peck I. Organization of the Nervous System (pp 222-224) Nervous system- the master controlling and communicating system of the body 3 functions: 1. sensory receptors to monitor changes occurring inside & outside body stimuli- changes sensory input- gathered information 2. processes and interprets the sensory input integration- nervous system makes decisions about what should be done 3. effects a response by activating muscles or glands (effectors) via motor output Regulating and Maintaining Homeostasis nervous system - fast-acting control via electrical impulses endocrine system- slow-acting control via hormones release into the blood Structural Classification (p 223) 2 subdivisions: Central Nervous System Peripheral Nervous System 1. Central Nervous System (CNS) consists of: brain spinal cord functions: integrating center.........interpret incoming sensory information command center..........issue instructions based on past experience & current conditions 2. Peripheral Nervous System (PNS) consists of: nerves 2 types: cranial nerves- carry impulses to and from the brain spinal nerves- carry impulses to and from the spinal cord ganglia- groups of nerve cell bodies function: communication lines, linking all parts of the body Functional Classification (pp 223-224) 2 only deals with peripheral nervous system (PNS) 1. Sensory (Afferent) Division- nerve fibers that carry impulses to the CNS from sensory receptors located throughout body sensory fibers types: 1. somatic sensory fibers- delivering impulses from the skin, skeletal muscles, & joints 2. visceral sensory fibers- transmitting impulses from the visceral organs 2. Motor (Efferent) Division- nerve fibers that carry impulses from the CNS to effector organs ossicles and glands, bringing about a motor response 2 types: 1. -

Laboratory Administration of Transcutaneous Auricular Vagus Nerve Stimulation (Tavns): Technique, Targeting, and Considerations
Journal of Visualized Experiments www.jove.com Video Article Laboratory Administration of Transcutaneous Auricular Vagus Nerve Stimulation (taVNS): Technique, Targeting, and Considerations Bashar W. Badran*1,2,3, Alfred B. Yu*2, Devin Adair1, Georgia Mappin3, William H. DeVries3, Dorothea D. Jenkins4, Mark S. George3,5,6, Marom Bikson1 1 Department of Biomedical Engineering, City College of New York 2 U.S. Army Research Laboratory, Aberdeen Proving Ground 3 Brain Stimulation Laboratory, Department of Psychiatry, Medical University of South Carolina 4 Department of Pediatrics, Medical University of South Carolina 5 Department of Neurology, Medical University of South Carolina 6 Ralph H. Johnson VA Medical Center * These authors contributed equally Correspondence to: Bashar W. Badran at [email protected] URL: https://www.jove.com/video/58984 DOI: doi:10.3791/58984 Keywords: Neuroscience, Issue 143, taVNS, tVNS, VNS, Transcutaneous Auricular Vagus Nerve Stimulation, vagus nerve stimulation, ear stimulation Date Published: 1/7/2019 Citation: Badran, B.W., Yu, A.B., Adair, D., Mappin, G., DeVries, W.H., Jenkins, D.D., George, M.S., Bikson, M. Laboratory Administration of Transcutaneous Auricular Vagus Nerve Stimulation (taVNS): Technique, Targeting, and Considerations. J. Vis. Exp. (143), e58984, doi:10.3791/58984 (2019). Abstract Non-invasive vagus nerve stimulation (VNS) may be administered via a novel, emerging neuromodulatory technique known as transcutaneous auricular vagus nerve stimulation (taVNS). Unlike cervically-implanted VNS, taVNS is an inexpensive and non-surgical method used to modulate the vagus system. taVNS is appealing as it allows for rapid translation of basic VNS research and serves as a safe, inexpensive, and portable neurostimulation system for the future treatment of central and peripheral disease.