Fish Kills, Bottom-Water Hypoxia, and the Toxic Pfiesteria Complex in The
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Trout Fishing in Hillsdale July, 2012 Guy Winig, Member Hillsdale Conservation Advisory Council
Roe Jan Brown-released to fight another day. Trout Fishing in Hillsdale July, 2012 Guy Winig, member Hillsdale Conservation Advisory Council Trout are our canaries in a coalmine. They are an excellent indicator of stream health, requiring clean, cold, well-oxygenated water to thrive. Without very high quality surface waters, trout just cannot survive. Hillsdale is fortunate to have several miles of trout streams including the Roeliff Jansen Kill, Taconic Creek, Agawamuck Creek and Green River with numerous smaller and unnamed tributaries contributing to them. Our streams and their residents endure many perils including natural flood and drought cycles as well as man made impacts like pollution, invasive species, barriers and over –fishing. Erosion adds silt that smothers aquatic food sources and gravel spawning beds. Introduced Didymo algae (a.k.a. ‘Rock Snot’) now found in the Green River, also chokes the streambed. Loss of vegetation exposes water to heating and eliminates important breeding, feeding and cover areas for trout. Readers may recall the massive fish kill that resulted from the release of manure into the Roe Jan in the 90’s or tales of unscrupulous ‘sportsmen’ using small explosives and chlorine to kill all the fish in a segment of stream. Maybe the most harmful and insidious are non-point sources of pollution such as chemicals, fertilizers and heated runoff from parking lots, roads, roofs, crop fields and lawns. In 2009 and 2010, members of the community with help from the Columbia-Greene chapter of Trout Unlimited and the N.Y.S.D.E.C. Trees for Tributaries program planted several hundred small trees and shrubs on the banks and flood plain of the Roe Jan Kill through the Roe Jan Park in an effort to stabilize banks, introduce vegetative cover and provide shade to maintain cool stream temperatures. -

Localized Plankton Blooms and Jubilees on the Gulf Coast Gordon Gunter Gulf Coast Research Laboratory
Gulf Research Reports Volume 6 | Issue 3 January 1979 Localized Plankton Blooms and Jubilees on the Gulf Coast Gordon Gunter Gulf Coast Research Laboratory Charles H. Lyles Gulf Coast Research Laboratory DOI: 10.18785/grr.0603.12 Follow this and additional works at: http://aquila.usm.edu/gcr Part of the Marine Biology Commons Recommended Citation Gunter, G. and C. H. Lyles. 1979. Localized Plankton Blooms and Jubilees on the Gulf Coast. Gulf Research Reports 6 (3): 297-299. Retrieved from http://aquila.usm.edu/gcr/vol6/iss3/12 This Short Communication is brought to you for free and open access by The Aquila Digital Community. It has been accepted for inclusion in Gulf and Caribbean Research by an authorized editor of The Aquila Digital Community. For more information, please contact [email protected]. Gulf Research Reports, Vol. 6,NO. 3,291-299, 1919. LOCALIZED PLANKTON BLOOMS AND JUBILEES ON THE GULF COAST GORDON GUNTER AND CHARLES H. LYLES Gulf Coast Research Laboratory, Ocean Springs, Mississippi 39564 and Gulf States Marine Fisheries Commission, Ocean Springs, Mississippi 39564 ABSTRACT The writers describe various small types of plankton blooms such as those occurring in boat slips, the head of a large bayou and a strip type bloom of Chaeroceras on the Gulf beach. Oyster kills from “poison water” draining off of marshes are said to be caused by plankton bloom. Small “jubilees” are said to be caused by localized blooms and one of these is described as it occurred. In November 1938, Dr. Margaretha Brongersma-Sanders of billions of Chaetoceras sp., a common open sea diatom. -

FISHING NEWSLETTER 2020/2021 Table of Contents FWP Administrative Regions and Hatchery Locations
FISHING NEWSLETTER 2020/2021 Table of Contents FWP Administrative Regions and Hatchery Locations .........................................................................................3 Region 1 Reports: Northwest Montana ..........................................................................................................5 Region 2 Reports: West Central Montana .....................................................................................................17 Region 3 Reports: Southwest Montana ........................................................................................................34 Region 4 Reports: North Central Montana ...................................................................................................44 Region 5 Reports: South Central Montana ...................................................................................................65 Region 6 Reports: Northeast Montana ........................................................................................................73 Region 7 Reports: Southeast Montana .........................................................................................................86 Montana Fish Hatchery Reports: .......................................................................................................................92 Murray Springs Trout Hatchery ...................................................................................................................92 Washoe Park Trout Hatchery .......................................................................................................................93 -
Fish Kills in NSW Frequently Asked Questions
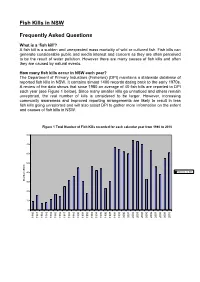
Fish Kills in NSW Frequently Asked Questions What is a ‘fish kill’? A fish kill is a sudden and unexpected mass mortality of wild or cultured fish. Fish kills can generate considerable public and media interest and concern as they are often perceived to be the result of water pollution. However there are many causes of fish kills and often they are caused by natural events. How many fish kills occur in NSW each year? The Department of Primary Industries (Fisheries) (DPI) maintains a statewide database of reported fish kills in NSW. It contains almost 1400 records dating back to the early 1970s. A review of the data shows that since 1980 an average of 40 fish kills are reported to DPI each year (see Figure 1 below). Since many smaller kills go unnoticed and others remain unreported, the real number of kills is considered to be larger. However, increasing community awareness and improved reporting arrangements are likely to result in less fish kills going unreported and will also assist DPI to gather more information on the extent and causes of fish kills in NSW. Figure 1 Total Number of Fish Kills recorded for each calendar year from 1980 to 2010 80 70 60 50 40 Total no. of kills Number of kills 30 20 10 0 1980 1981 1982 1983 1984 1985 1986 1987 1988 1989 1990 1991 1992 1993 1994 1995 1996 1997 1998 1999 2000 2001 2002 2003 2004 2005 2006 2007 2008 2009 2010 What species of fish are affected? Over 100 species of fish, including finfish, molluscs and crustaceans, have been reported in fish kills in NSW since records began. -

Lower Darling River Fish Death Event, Menindee 2018/19
• Fish Death Interim Investigation Report • Lower Darling River Fish Death Event, Menindee 2018/19 Executive Summary A NSW Department of Primary Industries (DPI) investigation has found that it is likely a lack of dissolved oxygen in the Lower Darling River caused large-scale fish deaths in early January and a smaller incident in the same area in December 2018. DPI scientists consulted with Murray-Darling Basin experts in biochemistry and algal ecology and found that both fish deaths were likely to have been caused by several related and compounding factors resulting in low oxygen in the river, including: High temperatures and low or no water flow conditions led to thermal stratification. A situation in which warmer surface layer (~1m) of water sat above a cooler deeper layer (2-3m) of water with very low dissolved oxygen. These conditions were conducive to blue-green algal blooms in the surface layers. Rainfall on December 15 2018 and an associated drop in temperature appears to have mixed water layers, which resulted in very low dissolved oxygen throughout the water column. Another substantial drop in air temperatures from 46 degrees to 28 degrees on January 4 and 5 associated with cold fronts passing through the region again caused layers of water with different dissolved oxygen levels to mix, reducing the overall dissolved oxygen available. High algal content in stock and domestic flow releases drawn from Lake Pamamaroo, which increased oxygen demand and consumption, further reduced dissolved oxygen available to fish. However, it is not possible to determine unequivocally the cause of the fish deaths in Menindee without comprehensive data on the conditions in the Darling River immediately prior to the first sighting of dead fish DPI will continue to remain on high alert for potential future fish deaths in the Lower Darling River, and have commenced the deployment of aeration devices. -

How to Prevent Pesticide-Related Fish Kills a Guide for Homeowners and Pesticide Applicators
Clemson University Department of Pesticide Regulation How to Prevent Pesticide-Related Fish Kills A Guide for Homeowners and Pesticide Applicators The Clemson University Department of Pesticide Regulation (DPR) is responsible for education and enforcement of laws related to safe pesticide use. Part of this involves preventing the loss of natural resources in South Carolina’s coastal and fresh water ar- eas while maintaining efficient control of insects and other pests. Who is Responsible for Investigating Pesticide-Related Fish Kills? Each year, inspectors with the Department of Pesticide Regula- Department of tion investigate pesticide-related fish kills in South Carolina lakes, streams, ponds, and salt-water inlets. A fish kill comprises Pesticide Regulation large numbers of dead or dying fish, not just one or two. 511 Westinghouse Road Locations of these kills range from farm ponds, ponds located on or adjacent to golf courses, apartment buildings and condomini- Pendleton, SC 29670 ums, as well as salt water inlets and tributaries of SC rivers. Croaker, mullets, and bluegills are among the most common 864.646.2150 South Carolina fish reported. www.clemson.edu/dpr The Department of Pesticide Regulation works together with other State agencies and the agricultural community to ensure that the continued availability of effective pesticides is not compromised by unwise, illegal, or inappropriate use. In the late 1980s and early 1990s, for example, the Department of Pesticide Regulation fought for and maintained a Administration special pesticide registration to replace an environmentally harmful pesticide with a milder one. This eliminated a series of fish kills that had plagued coastal tomato growers in South Carolina for years. -

The Resources Agency of California Department of Fish and Game Marine Resources Operations
Report for the month of August 1962 Item Type monograph Publisher California Department of Fish and Game, Marine Resources Operations Download date 23/09/2021 13:42:02 Link to Item http://hdl.handle.net/1834/18812 '. THE RESOURCES AGENCY OF CALIFORNIA DEPARTMENT OF FISH AND GAME MARINE RESOURCES OPERATIONS REPORT FOR THE MONTH OF AUGUST 1962 ACCOMPLISHMENTS MRO biologists have tagged and released nearly 1,000 bluefin tuna and 350 sand bass during the past two months. Recoveries from these could provide answers to mysteries that have puzzled fishermen and biologists for many decades: where are the bluefin tuna during the 6 or 8 months they are not in our waters, do they travel to Japan or Australia each year, where do they congregate to spawn, and how East do they grow; where did the tremen dous population of adult sand bass that's in our waters this summer come from, will it move away when winter comes, will it be fished out, and so on? HIGHLIGHTS The Area A shrimp fishery 'surged and nearly 500,000 pounds were landed at Crescent City and Eureka during the month. The success of the commercial albacore fishery was reflected in a price cut by the canners late in August -- the second for the season -- to $310 per ton, $90 less than the season's starting price. ~~~fr~ tutia, 960 of them, were tagged during a cooperative cruise with the U.S. Bureau of Commercial Fisheries aboard their chartered purse:;seiner .. Westpoint. The predicted sardine catch for the 1962-63 sardine season, released at the MRC meeting August 22, was a meager 5,000 to 15,000 tons. -

Tropical Reef-Fish Disease Outbreaks and Mass Mortalities in Florida, USA: What Is the Role of Dietary Biological Toxins?
DISEASES OF AQUATIC ORGANISMS Vol. 22: 83-100, 1995 Published June 15 1 Dis aquat Org Tropical reef-fish disease outbreaks and mass mortalities in Florida, USA: what is the role of dietary biological toxins? Jan H. Landsberg Florida Marine Research Institute, Florida Department of Environmental Protection, 100 Eighth Avenue Southeast, St. Petersburg. Florida 33701-5095, USA ABSTRACT. From November 1993 to February 1994, heavy mostallties of troplcal reef fish were reported in the Palm Beach area on Florida's southeast coast and the Islamorada area in the upper Florida Keys. Severely affected fish were typically adult herbivores or omnivores such as the angel- fishes Pomacanthus paru and P. arcuatus, the rock beauty Holacanthus tricolor, the cherubfish Centropyge argi, the princess parrotfish Scarus taenlopterus, the blue chsomis Chrornis cyaneus, the balloonfish Diodon holocanthus, the whitespotted f~lef~shCantherhines rnacroceros, the doctorfish Acanthurus chirurgus, and less severely affected, the reef butterflyflsh Chaetodon sedentarius and the foureye butterflyfish C. capistratus. Diseased fish typically had lesions on the anterior part of the head, ulcerated body sores, fin and tail rot, and a heavy mucus coating on the body surface. Protis- tan parasites such as Brooklynella hostilis, Uronema marinurn, and amoebae were common. Turbel- larians and bacterial infestations were also detected. There is, however, no evidence that these potential pathogens are the principal cause of the disease syndrome and resultant fish mortality - they may be secondary invaders of fish whose health has already been compromised. The possi- bility is considered that biological toxins from macroalgae such as Caulerpa spp., or from benthic dinoflagellates such as Garnbierdiscus toxicus may act as principal stressors that influence reef-fish health. -

An Oceanographic, Meteorological, and Biological 'Perfect Storm'yields
Vol. 468: 231–243, 2012 MARINE ECOLOGY PROGRESS SERIES Published November 14 doi: 10.3354/meps09927 Mar Ecol Prog Ser An oceanographic, meteorological, and biological ‘perfect storm’ yields a massive fish kill Beth A. Stauffer1,3,*, Alyssa G. Gellene1, Astrid Schnetzer1,4, Erica L. Seubert1, Carl Oberg2, Gaurav S. Sukhatme2, David A. Caron1 1Department of Biological Sciences and 2Department of Computer Science, University of Southern California, Los Angeles, California 90089, USA 3Present address: Lamont-Doherty Earth Observatory at Columbia University, Biology and Paleo-Environment, Palisades, New York 10964, USA 4Present address: Department of Marine, Earth, and Atmospheric Sciences, North Carolina State University, Raleigh, North Carolina 27695, USA ABSTRACT: Mass mortality events are ephemeral phenomena in marine ecosystems resulting from anthropogenically enhanced and natural processes. A fish kill in King Harbor, Redondo Beach, California, USA, in March 2011 killed ~1.54 × 105 kg of fish and garnered international attention as a marine system out of balance. Here, we present data collected prior to, during, and following the event that describe the oceanographic conditions preceding the event, spatial extent of hypoxia (dissolved oxygen < 1.4 ml l−1), and subsequent recovery of the harbor. In situ sensors within the harbor revealed rapid decreases in dissolved oxygen in surface waters from 7 to 9 March 2011, coincident with the mortality event on 8 March. Continuous observations provided evidence that respiration of a large population of fish within the harbor, potentially exacerbated by an incursion of upwelled low-oxygen water, resulted in significant oxygen reduction in the har- bor and ultimately caused mortality of the fish population. -

Impacts of Land and \Mater Tlse on Fish and Their Habitat Part I
Alasha Habitat Management Guide Impacts of land and \Mater tlse on Fish and Their Habitat Part I Produced by State of Alasha Department of Fish and Game Division of Habitat Iuneau, Alasha 1986 Olgge BYTHE ATASKADEpAPTMENT OF FISHAND CIAME Contents Achnowledgements v General Introduction 0verview of the Habitat Management Guides Project 3 Introduction to Part 1 7 Purpose 7 Approach 7 Organization of the Volume 8 Users Guide 10 Methods 11 Cross-reference of Activities 13 Activiry Index Marine Fish and Shellfish 31 Freshwater Fish 61 Impact Index Marine Fish and Shellfish 119 Freshwater Fi sh i51 Appendices A. Di rectory of Reviewers and Contributors 207 B. Land and Water Uses and Development Types 208 C. Descriptions of Activities 209 D. Fish Impact Categories 2L0 E. Correction Form zLl F. Additional References 213 Map 1. The six regions of the Alaska Habitat Management Guides 5 444 Figtrres 1. Types of narratives and maps produced by the Alaska Habitat Management Guides Project 6 t. General hierarch'ical relationships of land and water uses, activ'ities and impacts 9 Itables 1. Activities involved'in each land or water use or development type 10 2. Sunmary of the I iterature for defined activi tyl impact categories: marine fish and shellfish 32 3. Summary of the I iterature for defined activity/impact categories: freshwater fi sh 62 4. Summary of the I iterature for defined activity/impact categories: marine fish and shellfish I20 5. Sununary of the I iterature for defined activity/impact categories: freshwaier fish L52 Achnowledgements This project is under the djrection of the Conmissioner of the Department of j Di the Divi s'ion of Habi tat F sh and- Game, Don W. -

Winter Fish Kills Fact Sheet
CONNECTICUT DEPARTMENT OF ENERGY & ENVIRONMENTAL PROTECTION Inland Fisheries Division Habitat Conservation and Enhancement Program Winter Fish Kills Fact Sheet WHAT IS A FISH KILL? An event where large numbers of fish die, indicating a problem in the body of water. Fish kills can be caused by a variety of factors including dissolved oxygen depletion, extreme water temperatures, fish diseases or introduction of pollutants. Most fish kills are natural events. Example of Winter Kill in a Connecticut Pond WINTER FISH KILLS: Are generally caused by a depletion of dissolved oxygen. Winter kills occur most frequently in very shallow, nutrient rich ponds that are subject to abundant growth of aquatic plants and algae. Conditions conducive to winterkill arise when heavy snow cover over ice inhibits sunlight penetration, thereby preventing aquatic plants and algae from producing oxygen via photosynthesis. This process is the sole means of oxygen creation under ice-covered ponds. Also, the greater the load of dead and decaying plants and other organic material, the more rapid the loss of oxygen and the more quickly fish can be stressed or killed by low dissolved oxygen levels (See Figure 1). Fish typically die during the winter and are only observed following ice-out. DEEP • Inland Fisheries Division • Habitat Conservation and Enhancement Program 1 Revised 03/01/11 HOW TO PREVENT: Generally, a thaw that occurs in or around Figure 1. The Winter Kill Process February provides enough sunlight to recharge oxygen supply by means of photosynthesis and melt waters. However, for a harsh winter with a lot of snow and an extended period of ice cover, one of the best things to do is clear snow from several areas of the pond to allow sunlight to penetrate through the ice. -

July 2020 Fish Kills What Is a Fish Kill? When a Lot of Fish Die in a Localized
July 2020 Fish Kills Dead fish floating amidst algal bloom. California Department of Fish and Wildlife. What is a fish kill? When a lot of fish die in a localized area, it is often referred to as a fish kill or die-off. You will often see dead fish and other aquatic organisms floating on the water’s surface when there is a fish kill. The water around the fish may be scummy, discolored, smell foul, or otherwise appear contaminated (see concerns about Algal Blooms and Discolored and/or Foul-Smelling Water). What causes a fish kill? There are many reasons why you may see a fish kill (see the Missouri Department of Conservation page on fish kills), but the most common cause is low oxygen concentration in water. Nutrient pollution from CAFOs can produce algal blooms which over-consume oxygen and produce “dead zones” in the water where you may observe these fish kills. Why should you be worried? Fish kills are generally indicative of other contamination concerns. Disease and other natural events can lead to a fish kill, but according to the Missouri Department of Conservation, “municipal and agricultural waste water have been the leading sources of fish kills in Missouri since the 1940s” (MDC). Therefore, if you see a fish kill it may be evidence of pollution concerns and a warning sign to test your water. What can you do? July 2020 1. Call the Missouri Department of Natural Resources Environmental Emergency Response (EER) Section at 573-634-2436. The DNR considers fish kills an environmental emergency: an event which “poses an immediate threat to the public health or the well-being of the environment”.