Information for the User Solpadeine Soluble Tablets Paracetamol
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-
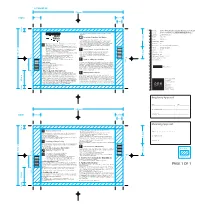
Solpadeine Max Tabs 03
File title: 9034_SOMT01240812v1_Solpadeine Max tabs 20s-30s PIL v3F • If you take a painkiller for headaches for more than 3 days it can make Item No.: 11000000010649 PROSP SOLPADEINA MAX TABLETAS UK 1212 them worse. Last edit: 22nd November 2013 Do not take Solpadeine Max Tablets: Brand: Solpadeine Tablets Variant: MAX Tablets • If you have ever had an allergic reaction to paracetamol, codeine, other opioid painkillers or to any of the other ingredients (listed in Section 6) Component: Leaflet • If you are taking other medicines containing paracetamol or codeine • For pain relief in children and adolescents (0-18 years of age) after removal • This medicine is for the short term treatment of acute moderate pain Market/s: UK of their tonsils or adenoids due to obstructive sleep apnoea syndrome when other painkillers have not worked. • If you know that you metabolise very rapidly codeine into morphine • You should only take this product for a maximum of 3 days at a time. If Dimensions: 150 x 100mm (Drw No. L613D005) • If you are breastfeeding. you need to take it for longer than 3 days you should see your doctor or pharmacist for advice. Printer: Santiago Gonzalez • This medicine contains codeine which can cause addiction if you take it Ask your doctor before you take this medicine: continuously for more than 3 days. This can give you withdrawal Substrate: Paper symptoms from the medicine when you stop taking it. • If you have liver or kidney disease, including alcoholic liver disease Tablets EAN Code: N/A • If you take this medicine for headaches for more than 3 days it can make • If you have bowel problems including blockage of your bowel them worse. -

Glaxosmithkline Plc Annual Report for the Year Ended 31St December 2000
GlaxoSmithKline 01 GlaxoSmithKline plc Annual Report for the year ended 31st December 2000 Contents Report of the Directors 02 Financial summary 03 Joint statement by the Chairman and the Chief Executive Officer 05 Description of business 29 Corporate governance 37 Remuneration report 47 Operating and financial review and prospects 69 Financial statements 70 Directors’ statements of responsibility 71 Report by the auditors 72 Consolidated statement of profit and loss 72 Consolidated statement of total recognised gains and losses 74 Consolidated statement of cash flow 76 Consolidated balance sheet 76 Reconciliation of movements in equity shareholders’ funds 77 Company balance sheet 78 Notes to the financial statements 136 Group companies 142 Principal financial statements in US$ 144 Financial record 153 Investor information 154 Shareholder return 156 Taxation information for shareholders 157 Shareholder information 158 Share capital 160 Cross reference to Form 20-F 162 Glossary of terms The Annual Report was approved by the Board 163 Index of Directors on 22nd March 2001 and published on 12th April 2001. Contact details 02 GlaxoSmithKline Financial summary 2000 1999 Increase Business performance £m £m CER % £ % Sales 18,079 16,164 9 12 Trading profit 5,026 4,378 12 15 Profit before taxation 5,327 4,708 11 13 Earnings/Net income 3,697 3,222 13 15 Earnings per Ordinary Share 61.0p 52.7p 14 16 Total results Profit before taxation 6,029 4,236 Earnings/Net income 4,154 2,859 Earnings per Ordinary Share 68.5p 46.7p Business performance: results exclude merger items and restructuring costs; 1999 sales and trading profit exclude the Healthcare Services businesses which were disposed of in 1999. -

Malta Medicines List April 08
Defined Daily Doses Pharmacological Dispensing Active Ingredients Trade Name Dosage strength Dosage form ATC Code Comments (WHO) Classification Class Glucobay 50 50mg Alpha Glucosidase Inhibitor - Blood Acarbose Tablet 300mg A10BF01 PoM Glucose Lowering Glucobay 100 100mg Medicine Rantudil® Forte 60mg Capsule hard Anti-inflammatory and Acemetacine 0.12g anti rheumatic, non M01AB11 PoM steroidal Rantudil® Retard 90mg Slow release capsule Carbonic Anhydrase Inhibitor - Acetazolamide Diamox 250mg Tablet 750mg S01EC01 PoM Antiglaucoma Preparation Parasympatho- Powder and solvent for solution for mimetic - Acetylcholine Chloride Miovisin® 10mg/ml Refer to PIL S01EB09 PoM eye irrigation Antiglaucoma Preparation Acetylcysteine 200mg/ml Concentrate for solution for Acetylcysteine 200mg/ml Refer to PIL Antidote PoM Injection injection V03AB23 Zovirax™ Suspension 200mg/5ml Oral suspension Aciclovir Medovir 200 200mg Tablet Virucid 200 Zovirax® 200mg Dispersible film-coated tablets 4g Antiviral J05AB01 PoM Zovirax® 800mg Aciclovir Medovir 800 800mg Tablet Aciclovir Virucid 800 Virucid 400 400mg Tablet Aciclovir Merck 250mg Powder for solution for inj Immunovir® Zovirax® Cream PoM PoM Numark Cold Sore Cream 5% w/w (5g/100g)Cream Refer to PIL Antiviral D06BB03 Vitasorb Cold Sore OTC Cream Medovir PoM Neotigason® 10mg Acitretin Capsule 35mg Retinoid - Antipsoriatic D05BB02 PoM Neotigason® 25mg Acrivastine Benadryl® Allergy Relief 8mg Capsule 24mg Antihistamine R06AX18 OTC Carbomix 81.3%w/w Granules for oral suspension Antidiarrhoeal and Activated Charcoal -
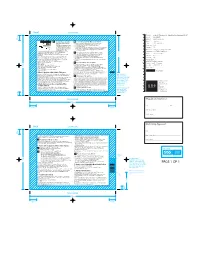
Solpadeine Max Soluble Tabs 03
FRONT LEADING EDGE File title: 9034_SOLPM00890812v1_Solpadeine Max Soluble tabs PIL v5F Item No.: 40L564D(OPI) Last edit: 22nd November 2013 8.2mm This medicine is for the short term Brand: Solpadeine treatment of acute moderate pain Do not take Solpadeine Max Soluble Tablets: when other painkillers have not If you have ever had an allergic reaction Variant: MAX soluble tablets worked. You should only take this product for taking other medicines containing paracetamol. Component: Leaflet a maximum of 3 days at a time. If under 12 years: you need to take it for longer than 3 For pain relief in children and adolescents (0-18 years of age) after removal Market/s: UK of their tonsils or adenoids due to obstructive sleep apnoea syndrome days you should see your doctor Dimensions: 150 x 105mm (Drw No. OTC.LT.008) or pharmacist for advice. If you know that you metabolise very rapidly codeine into morphine If you are breastfeeding. This medicine contains codeine, which can cause addition if you take it 64.2mm Copy position: 1 OUTSIDE, 5 INSIDE print continuously for more than 3 days. This can give you withdrawal Ask your doctor before you take this medicine: symptoms from the medicine when you stop taking it. Printer: Chesapeake St Pierre If you take this medicine for headaches for more than 3 days, it can make liver kidney disease alcoholic liver disease them worse. bowel problems Substrate: Paper Please read right through this leaflet before you start using this medicine. operation to remove your gall bladder intolerance to EAN Code: N/A some sugars controlled sodium diet. -

Oesophageal Stricture Associated with Emepronium Bromide Therapy 1
Postgrad Med J: first published as 10.1136/pgmj.58.675.43 on 1 January 1982. Downloaded from Postgraduate Aledical Journal (January 1982) 58, 43-44 Oesophageal stricture associated with emepronium bromide therapy 1. W. FELLOWS A. L. OGILVIE M.B., M.R.C.P. M.B., M.R.C.P. M. ATKINSON M.D., F.R.C.P. University Hospital, Queen's Medical Centre, Nottingham Summary Barium swallow showed a small fixed hiatus Emepronium bromide, a drug used to control urinary hernia with a stricture above it and an ulcer crater frequency, has been reported as causing oesophageal therein. ulceration but not stricture formation. This paper Endoscopy showed a 5-mm diameter benign presents 3 cases in which the use of emepronium stricture at 33 cm from the alveolar margin; the bromide preceded development of an oesophageal stricture was 1 cm long and was ulcerated. Below it stricture and suggests that the drug played a causative was a hiatus hernia. The stricture was dilated, the role. emepronium stopped and she was given cimetidine and Asilone gel. One further dilatation was needed Case reports 4 months later but she has swallowed well for the Case I last 6 months. copyright. A 50-year-old woman, with a 6-year history of multiple sclerosis causing spastic paraparesis of the Case 3 lower limbs, presented with a 2-month history of A 74-year-old woman presented with progressive dysphagia for solids, retrosternal pain and 12-7 kg dysphagia for solids for a few weeks and mild weight loss. She was taking baclofen, carbamazepine, heartburn. -

Annual Report 2008 Find out More About GSK Online…
Do more, feel better, live longer Grow Deliver Simplify Annual Report 2008 Find out more about GSK online… www.gsk.com Website GlaxoSmithKline’s website www.gsk.com gives additional information on the Group. Information made available on the website does not constitute part of this Annual Report. Notice regarding limitations on Director liability under English Law Under the UK Companies Act 2006, a safe harbour limits the liability of Directors in respect of statements in and omissions from the Report of the Directors contained on pages 12 to 98. Under English law the Directors would be liable to the company (but not to any third party) if the Report of the Directors contains errors as a result of recklessness or knowing misstatement or dishonest concealment of a material fact, but would not otherwise be liable. Report of the Directors Pages 12 to 98 inclusive consist of a Report of the Directors that has been drawn up and presented in accordance with and in reliance upon English company law and the liabilities of the Directors in connection with that report shall be subject to the limitations and restrictions provided by such law. Cautionary statement regarding forward-looking statements The Group’s reports filed with or furnished to the US Securities and Exchange Commission (SEC), including this document and written information released, or oral statements made, to the public in the future by or on behalf of the Group, may contain forward-looking statements. Forward-looking statements give the Group’s current expectations or forecasts of future events. A shareholder can identify these statements by the fact that they do not relate strictly to historical or current facts. -

Self Help Gastrointestinal System Irritable Bowel Syndrome (IBS)
Self Help This guide is to help you see which conditions you can safely try treating yourself or with the advise of your pharmacist. For information on all conditions listed below (and many more) see www.NHS.uk - HEALTH A-Z Prices: The guide below gives an indication of cost. At the time of writing (July 2019) the cost of a single medication on prescription in England was £9 (see www.NHS.UK for up to date charges) £ ££ £££ 50p - £2 £2 - £6 £6-£10 Gastrointestinal System Dyspepsia or acid reflux (Indigestion or heart burn) • Discomfort in the upper abdomen or chest often after eating. Associated with feeling sick, full or bloated. Bringing up food or bitter tasting liquids. See GP if : keep getting indigestion • are in bad pain • are 55 or older • have lost a lot of weight without meaning to • have difficulty swallowing (dysphagia) • keep vomiting • have iron deficiency anaemia • feel like you have a lump in your stomach • have bloody vomit or poo These symptoms can be a sign of something more serious. Name of Medicine Cost Gaviscon® Advance Liquid £££ Gaviscon ®Advance Tablets ££ Ranitidine 75mg Tablets £ Irritable Bowel Syndrome (IBS) A common condition affecting the digestive system causing, stomach cramps, bloating, diarrhea and/or constipation. Often a lifelong problem. For more information see https://www.bda.uk.com/foodfacts/IBSfoodfacts.pdf See GP if there is bleeding when passing stool, a family history of Inflammatory bowel disease or coeliacs, or a family history of ovarian cancer, or if the condition is persistent and intrusive. Produced by: The Medicine Box and The University Medical Centre Updated: August 2019 If you are over 60 and develop new symptoms like this see your GP. -

Glaxosmithkline Annual Report 2010
Do more, feel better, live longer GlaxoSmithKline Annual Report 2010 Contents Business review P08–P57 Business review 2010 Performance overview 08 Research and development 10 Pipeline summary 12 Products, competition and intellectual property 14 Regulation 18 Manufacturing and supply 19 Business review World market 20 This discusses our financial and non-financial activities, GSK sales performance 21 resources, development and performance during 2010 Segment reviews 22 and outlines the factors, including the trends and the Responsible business 29 principal risks and uncertainties, which are likely to Financial review 2010 34 affect future development. Financial position and resources 41 Financial review 2009 47 Governance and remuneration Risk factors 53 This discusses our management structures and governance procedures. It also sets out the Governance and remuneration P58–P101 Governance and remuneration Governance and remuneration remuneration policies operated for our Directors and Our Board 58 Corporate Executive Team members. Our Corporate Executive Team 60 Governance and policy 64 Financial statements Dialogue with shareholders 69 The financial statements provide a summary of the Internal control framework 71 Group’s financial performance throughout 2010 and its Committee reports 74 position as at 31st December 2010. The consolidated Remuneration policy 84 financial statements are prepared in accordance with Director terms and conditions 91 IFRS as adopted by the European Union and also IFRS as Director and Senior Management remuneration 94 issued by the International Accounting Standards Board. Directors’ interests 96 Directors’ interests in contracts 101 Shareholder information This includes the full product development pipeline and discusses shareholder return in the form of dividends and share price movements. -
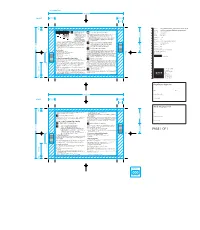
Solpadeine Plus Capsules
File title: 9034_SOLPC00940812v1_Solpadeine Plus capsules PIL v8F Item No.: 11000000010692 PROSP SOLPADEINA CAPSULAS UK 1212 ® • This medicine is for the short term treatment of acute moderate pain Do not take Solpadeine Plus Capsules: Last edit: 22nd November 2013 when other painkillers have not worked. • If you have ever had an allergic reaction to paracetamol, caffeine, codeine, other Brand: Solpadeine • You should only take this product for a opioid painkillers or to any of the other ingredients (listed in Section 6) maximum of 3 days at a time. If you • If you are taking other medicines containing paracetamol or codeine Variant: Plus Capsules need to take it for longer than 3 days • If you are under 12 years: you should see your doctor or Component: Leaflet pharmacist for advice. • For pain relief in children and adolescents (0-18 years of age) after removal of • This medicine contains codeine their tonsils or adenoids due to obstructive sleep apnoea syndrome Market/s: UK which can cause addiction if you • If you know that you metabolise very rapidly codeine into morphine take it continuously for more than 3 • If you are breastfeeding. Dimensions: 150 x 100mm (Drw No. L613D005) days. This can give you withdrawal symptoms from the medicine when you stop taking it. • If you take this medicine for headaches for more than 3 days it can make them worse. Ask your doctor before you take this medicine: Printer: Santiago Gonzalez Please read right through this leaflet before you start using this medicine. This medicine is available without prescription, but you still need to use Solpadeine Plus • If you have liver or kidney disease, including alcoholic liver disease Substrate: White Paper Capsules carefully to get the best results from them. -

GSK Annual Report 2002
The Impact of Medicines Do more, feel better, live longer Annual Report 2002 www.gsk.com Mission Our global quest is to improve the quality of human life by enabling people to do more, feel better and live longer. Our Spirit We undertake our quest with the enthusiasm of entrepreneurs, excited by the constant search for innovation. We value performance achieved with integrity. We will attain success as a world class global leader with each and every one of our people contributing with passion and an unmatched sense of urgency. Strategic Intent We want to become the indisputable leader in our industry. GlaxoSmithKline plc is an English public limited company. Its shares are listed on the London Stock Exchange and the New York Stock Exchange. GlaxoSmithKline plc acquired Glaxo Wellcome plc and SmithKline Beecham plc on 27th December 2000 by way of a scheme of arrangement for the merger of the two companies which became effective on 27th December 2000. This report is the Annual Report of GlaxoSmithKline plc for the year ended 31st December 2002. It comprises in a single document the Annual Report of the company in accordance with United Kingdom requirements and the Annual Report on Form 20-F to the Securities and Exchange Commission in the United States of America. A summary report on the year, the Annual Review 2002, intended for the investor not needing the full detail of the Annual Report, is produced as a separate document. The Annual Review includes the joint statement by the Chairman and the Chief Executive Officer, a summary review of operations, summary financial statements and a summary remuneration report. -

Latest List of Pharmacy Only Medicinal Products Not Subject to Medical Prescription This List Was Last Updated on Thursday, September 23, 2021
Latest list of pharmacy only medicinal products not subject to medical prescription This list was last updated on Thursday, September 23, 2021 Please Note The contents of the table are believed to be correct at the time of compilation. The HPRA makes no representations or warranties about and disclaim all liability for the content, accuracy, completeness or suitability of the information contained in the table for any purpose whatsoever and the information is made available to the public for information purposes only. For any conditions associated with this general sale classification please refer to the detailed product information on the Human Medicines Product listing on the HPRA website. Please note that at time of print some products listed below may not be currently marketed. Part 1. Latest list of pharmacy only medicinal products not subject to medical prescription Trade Name Licence Holder Licence Strength Dosage Form Active Number Ingredients A.Vogel Uva-ursi & A. Vogel Ireland TR2309/021/001 . Oral drops, solution Echinacea Cystitis Ltd, oral drops Abidec Multivitamin Chefaro Ireland PA1186/001/001 25 millilitre(s) Oral drops, solution -Vitamin a palmitate Oral Drops Solution DAC -Ergocalciferolci -Thiamine hydrochloride -Riboflavin sodium phosphate -Pyridoxine hydrochloride -Nicotinamide -Ascorbic acid Acic 5% Cream Rowex Ltd PA0711/017/001 5 percent Cream -ACICLOVIR Acidex Oral Pinewood PA0281/075/001 500mg+267mg+16 Oral suspension -Sodium alginate SuspensionSodium Laboratories Ltd, 0 milligram/ 10 -Sodium Alginate millilitre bicarbonate 500mg,Sodium -Calcium carbonate Bicarbonate 267mg,Calcium Carbonate 160mg/10ml Acnecide 5% w/w Galderma PA22743/001/001 5 percent Gel -Benzoyl peroxide Gel International S.A.S. -

Codeine Art. 31
26 February 2013 EMA/124076/2013 Patient Health Protection Annex I List of the names, pharmaceutical forms, strengths of the medicinal products, routes of administration, marketing authorisation holders in the member states for codeine containing medicinal products used for pain in children Article 31 of Directive 2001/83/EC resulting from pharmacovigilance data Procedure number: EMEA/H/A-31/1342 7 Westferry Circus ● Canary Wharf ● London E14 4HB ● United Kingdom Telephone +44 (0)20 7418 8400 Facsimile +44 (0)20 7418 8416 E -mail [email protected] Website www.ema.europa.eu An agency of the European Union © European Medicines Agency, 2013. Reproduction is authorised provided the source is acknowledged. Member Marketing Product name INN Strength Pharmaceutical Route of State in EEA authorisaton holder form administration Austria Mundipharma GmbH, Codidol retard 60 mg - Dihydrocodeine 60 mg prolonged- oral use Apollogasse 16 - 18 Filmtabletten release tablet 1070 Vienna Austria Austria Mundipharma GmbH, Codidol retard 90 mg - Dihydrocodeine 90 mg prolonged- oral use Apollogasse 16 - 18 Filmtabletten release tablet 1070 Vienna Austria Austria Mundipharma GmbH, Codidol retard 120 mg - Dihydrocodeine 120 mg prolonged- oral use Apollogasse 16 - 18 Filmtabletten release tablet 1070 Vienna Austria Austria G.L. Pharma GmbH, Dehace retard 60 mg - Dihydrocodeine 60 mg prolonged- oral use Schloßplatz 1 Filmtabletten release tablet 8502 Lannach Austria Austria G.L. Pharma GmbH, Dehace retard 90 mg - Dihydrocodeine 90 mg prolonged- oral use Schloßplatz