GSK Annual Report 2002
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Governance and Remuneration 2014
Governance & remuneration reportStrategic In this section Our Board 72 Our Corporate Executive Team 76 Chairman’s letter 78 Corporate governance framework 79 Board report to shareholders Oversight and stewardship in 2014 and future actions 80 remuneration & Governance Leadership and effectiveness 82 Committee reports Audit & Risk 86 Nominations 92 Corporate Responsibility 94 Remuneration report Chairman’s annual statement 96 Annual report on remuneration 97 2014 Remuneration policy report 119 Financial statements Financial Investor information Investor GSK Annual Report 2014 71 Our Board Strategic reportStrategic Diversity Experience International experience Composition Tenure (Non-Executives) % % % % Scientific 19 Global 75 Executive 19 Up to 3 years 39 % % % % Finance 31 USA 100 Non-Executive 81 3-6 years 15 % % % % Industry 50 Europe 94 Male 69 7-9 years 23 % % % EMAP 63 Female 31 Over 9 years 23 Sir Christopher Gent 66 Skills and experience Chairman Sir Christopher has many years of experience of leading global businesses and a track record of delivering outstanding performance Governance & remuneration & Governance Nationality in highly competitive industries. He was appointed Managing Director British of Vodafone plc in 1985 and then became its Chief Executive Officer Appointment date in 1997 until his retirement in 2003. Sir Christopher was also a 1 June 2004 and as Chairman Non-Executive Director of Ferrari SpA and a member of the British on 1 January 2005 Airways International Business Advisory Board. Committee membership External appointments Corporate Responsibility Sir Christopher is a Senior Adviser at Bain & Co. Committee Chairman, Nominations, Remuneration and Finance Sir Philip Hampton 61 Skills and experience Chairman Designate Prior to joining GSK, Sir Philip chaired major FTSE 100 companies including J Sainsbury plc. -

Annual Report 2013
Annual Report 2013 “ Being active and having a positive outlook on life is what keeps me going every day.” Overview of 2013 “ Our performance in 2013 was defined by remarkable &R D output and further delivery of sustained financial performance for our shareholders.” Please go to page 4 for more More at gsk.com Performance highlights £26.5bn £8.0bn £7.0bn £5.2bn Group turnover Core* operating profit Total operating profit Returned to shareholders 6 112.2p 112.5p 13% Major medicines approved Core* earnings per share Total earnings per share Estimated return on R&D investment 10 6 1st 1st Potential phase III study starts in 2014/15 Potential medicines with phase III data in Access to Medicines Index Pharmaceutical company to sign AllTrials expected 2014/15 campaign for research transparency Front cover story Betty, aged 65, (pictured) has Chronic “ Health is important to me, Obstructive Pulmonary Disease (COPD). She only has 25% lung capacity. This means I try to take care of my she finds even everyday tasks difficult, but medicines and inhaled oxygen allow her to health with all the tools live as normal a life as she can. Betty’s mindset I have and do the best is to stay busy and active, so every week she goes to rehab exercise classes. that I can with it.” COPD is a disease of the lungs that leads to Betty, COPD patient, damaged airways, causing them to become North Carolina, USA narrower and making it harder for air to get in and out. 210 million people around the world are estimated to have COPD. -
![Beecham Group PLC and Another V Triomed (Pty) Ltd [2002] 4 All SA 193 (SCA)](https://docslib.b-cdn.net/cover/9907/beecham-group-plc-and-another-v-triomed-pty-ltd-2002-4-all-sa-193-sca-369907.webp)
Beecham Group PLC and Another V Triomed (Pty) Ltd [2002] 4 All SA 193 (SCA)
Beecham Group PLC and another v Triomed (Pty) Ltd [2002] 4 All SA 193 (SCA) Division: Supreme Court of Appeal Date: 19 September 2002 Case No: 100/01 Before: Harms, Scott, Mpati, Conradie JJA and Jones AJA Sourced by: PR Cronje Summarised by: D.Harris Parallel Citation: 2003 (3) SA 639 (SCA) . Editor's Summary . Cases Referred to . Judgment . [1] Intellectual property Trade marks Registration of shape as trade mark Where shape is not intended to be used to distinguish owner's products from those of another, it cannot be regarded as a trade mark Application for expungement from trade marks register therefore allowed. Editor's Summary Both parties involved in the present matter were players in the pharmaceutical industry. The Appellants sought the upholding of a trade mark for the shape of a tablet, and a finding that the Respondent had infringed the trade mark. The Respondent imported a tablet with the same composition and shape as that of the Appellants. In the court a quo, the Respondent applied for the expungement from the trade mark register of the shape trade mark. The Appellants launched a counterapplication for relief for trade mark infringement. The latter application failed while that of the Respondent succeeded. Held Interested parties may apply to court for the removal of an entry wrongly made or remaining on the trade mark register. The first question posed by the Court was whether the Appellants' shape mark constituted a trade mark in terms of section 10(1) of the Trade Marks Act 194 of 1993 ("the Act"). -

Annual Review 2005
GS2184_Review_A\W2.qxd 7/3/06 4:58 pm Page fc1 Annual Review 2005 human being Do more, feel better, live longer GS2184_Review_A\W2.qxd 9/3/06 1:28 pm Page ifc2 01 An interview with Sir Christopher Gent, Chairman, and JP Garnier, Chief Executive Officer 05 Tachi Yamada, Chairman, Research & Development, Pharmaceuticals 06 Jean Stéphenne, President and General Manager, GSK Biologicals 08 John Clarke, President, Consumer Healthcare 11 David Stout, President, Pharmaceutical Operations 14 Performance highlights 15 Business operating review 18 The Board 19 The Corporate Executive Team 20 Summary remuneration report 23 Corporate governance 24 Responsibility statements 25 Summary financial statements 26 Summary information under US GAAP 27 Shareholder information 29 Chairman and CEO’s closing letter JP Garnier (left) and Sir Christopher Gent (right) GS2184_Review_A\W2.qxd 7/3/06 5:01 pm Page 01 “Discovering important medicines, eradicating diseases, improving the quality of people’s lives and making medicines available to a greater number of people. This is what we do – and what we do matters to people.” JP Garnier, Chief Executive Officer An interview with Sir Christopher Gent, Chairman and JP Garnier, Chief Executive Officer 2005: a year of success and progress “Thanks to the efforts of our employees around the company’s pipeline is one of the largest and most world, 2005 was a very successful year for GSK,” says promising in the industry, with 149 projects in clinical JP Garnier, Chief Executive Officer. “Not only was it development (as at the end of February 2006), our best year ever from a financial standpoint, we also including 95 new chemical entities (NCEs), 29 product made substantial progress with our pipeline of line extensions (PLEs) and 25 vaccines. -
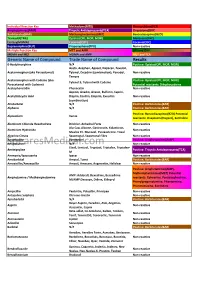
Cross Reaction Guide
Individual Reaction Key Methadone(MTD) Phencyclidine(PCP) Amphetamines(AMP) Tricyclic Antidepressants(TCA) Oxycodone(OXY) Barbiturates(BAR) Methamphetamines(MET) Benzodiazepines(BZO) Tramadol(TML) Opiates(OPI, MOP, MOR) Marijuana(THC) Ecstasy(MDMA) Cotinine(COT) Cocaine(COC) Buprenorphine(BUP) Propoxyphene(PPX) Non-reactive Multiple Reaction Key MET and AMP OPI and OXY MDMA and MET MDMA and AMP MET and TCA Generic Name of Compound Trade Name of Compound Results 6-Acetylmorphine N/A Positive: Opiates(OPI, MOP, MOR) Aceta, Acephen, Apacet, Dapacen, Feverall, Acetaminophen (aka Paracetamol) Tylenol, Excedrin (combination), Panadol, Non-reactive Tempra Acetaminophen with Codeine (aka Positive: Opiates(OPI, MOP, MOR) Tylenol 3, Tylenol with Codeine Paracetamol with Codeine) Potential reactants: Dihydrocodeine Acetophenetidin Phenacetin Non-reactive Aspirin, Anadin, Anasin, Bufferin, Caprin, Acetylsalicyclic Acid Disprin, Ecotrin, Empirin, Excedrin Non-reactive (combination) Allobarbital N/A Positive: Barbiturates(BAR) Alphenol N/A Positive: Barbiturates(BAR) Positive: Benzodiazepines(BZO) Potential Alprazolam Xanax reactants: Oxaprozin (Daypro), Sertraline Aluminum Chloride Hexahydrate Drichlor, Anhydrol Forte Non-reactive Alu-Cap, Alisone, Gastrocote, Kolanticon, Aluminum Hydroxide Non-reactive Maalox TC, Mucogel, Pyrogastrone, Topal Alverine Citrate Spasmonal, Spasmonal Fibre Non-reactive Amantadine Symmetrel Positive: Amphetamines(AMP) SpearesMedical.comAminopyrine N/A Non-reactive Elavil, Lentizol, Tryptizol, Triptafen, Triptafen- Amitriptyline -

In This Section
Strategic report In this section Chairman’s statement 2 CEO’s review 4 Business overview 6 The global context 8 Our business model 12 Our strategic priorities 14 How we performed 16 Risk management 18 Grow 20 Deliver 32 Simplify 44 Our financial architecture 48 Responsible business 50 Financial review 58 Strategic report Chairman’s statement Chairman’s statement To shareholders The value of the significant changes that have been made in recent years is evidenced in our performance this year “ Since Sir Andrew became It is clear from the following pages that Through the Audit & Risk Committee, we the Group made good progress against oversee the issues and challenges faced by CEO, the company has its strategy in 2013. management, and encourage the creation of an environment in which GSK can achieve The Board believes the business is seeing returned £30 billion its strategic ambitions in a responsible and the benefits of the significant changes the sustainable manner. to shareholders.” management team has driven over recent years to deliver sustainable growth, reduce risk and I have no doubt that commercial success is enhance returns to shareholders. directly linked to operating in a responsible way and which meets the changing expectations of The notably strong performance from the society. In this respect, the company continues R&D organisation in 2013 – with six major to adopt industry-leading positions on a range new product approvals in areas including of issues. respiratory disease, HIV and cancer – is critical to the longer-term prospects of the The announcement of plans during 2013 to Group. -
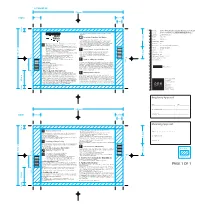
Solpadeine Max Tabs 03
File title: 9034_SOMT01240812v1_Solpadeine Max tabs 20s-30s PIL v3F • If you take a painkiller for headaches for more than 3 days it can make Item No.: 11000000010649 PROSP SOLPADEINA MAX TABLETAS UK 1212 them worse. Last edit: 22nd November 2013 Do not take Solpadeine Max Tablets: Brand: Solpadeine Tablets Variant: MAX Tablets • If you have ever had an allergic reaction to paracetamol, codeine, other opioid painkillers or to any of the other ingredients (listed in Section 6) Component: Leaflet • If you are taking other medicines containing paracetamol or codeine • For pain relief in children and adolescents (0-18 years of age) after removal • This medicine is for the short term treatment of acute moderate pain Market/s: UK of their tonsils or adenoids due to obstructive sleep apnoea syndrome when other painkillers have not worked. • If you know that you metabolise very rapidly codeine into morphine • You should only take this product for a maximum of 3 days at a time. If Dimensions: 150 x 100mm (Drw No. L613D005) • If you are breastfeeding. you need to take it for longer than 3 days you should see your doctor or pharmacist for advice. Printer: Santiago Gonzalez • This medicine contains codeine which can cause addiction if you take it Ask your doctor before you take this medicine: continuously for more than 3 days. This can give you withdrawal Substrate: Paper symptoms from the medicine when you stop taking it. • If you have liver or kidney disease, including alcoholic liver disease Tablets EAN Code: N/A • If you take this medicine for headaches for more than 3 days it can make • If you have bowel problems including blockage of your bowel them worse. -

With Nicotine Replacement Therapies in Smoking Cessation Vol
Health Technology Assessment Health Technology Health Technology Assessment 2008; Vol. 12: No. 2 2008; 12: No. 2 Vol. ‘Cut down to quit’ with nicotine replacement therapies in smoking cessation ‘Cut down to quit’ with nicotine replacement therapies in smoking cessation: a systematic review of effectiveness and economic analysis D Wang, M Connock, P Barton, A Fry-Smith, P Aveyard and D Moore Feedback The HTA Programme and the authors would like to know your views about this report. The Correspondence Page on the HTA website (http://www.hta.ac.uk) is a convenient way to publish your comments. If you prefer, you can send your comments to the address below, telling us whether you would like us to transfer them to the website. We look forward to hearing from you. February 2008 The National Coordinating Centre for Health Technology Assessment, Mailpoint 728, Boldrewood, Health Technology Assessment University of Southampton, NHS R&D HTA Programme Southampton, SO16 7PX, UK. HTA Fax: +44 (0) 23 8059 5639 Email: [email protected] www.hta.ac.uk http://www.hta.ac.uk ISSN 1366-5278 HTA How to obtain copies of this and other HTA Programme reports. An electronic version of this publication, in Adobe Acrobat format, is available for downloading free of charge for personal use from the HTA website (http://www.hta.ac.uk). A fully searchable CD-ROM is also available (see below). Printed copies of HTA monographs cost £20 each (post and packing free in the UK) to both public and private sector purchasers from our Despatch Agents. Non-UK purchasers will have to pay a small fee for post and packing. -

PDF (Irish Pharmacist January 2020)
EXCLUSIVE COVERAGE FROM THE RECENT IRISH MEDICATION SAFETY NETWORK ANNUAL CONFERENCE ABOUT-TURN WE LOOK AT WHAT MIGHT BE NEXT FOR PHARMACISTS FOLLOWING THE REVERSAL IN PROPOSED FEE CUTS DUMB AND DUMBER? ARE PHARMACISTS A LITTLE LACKING IN GREY MATTER WHEN IT COMES TO PRICING, ASKS FINTAN MOORE PLUS CLINICAL CONTENT: ASTHMA ❋ COPD ❋ EYE CARE ❋ SMOKING CESSATION TENDER CARE AT Every Change For topical use only. Cleanse and dry the affected area before applying. A copy of the summary of product characteristics is available upon request. The active ingredient in Caldesene Medicated Powder is Calcium Undecylenate 10% w/w, 20g, 55g, 100g pack size. For supply through general sale. PA 126/152/1 PA Holder: Clonmel Healthcare Ltd., Waterford Road, Clonmel, Co. Tipperary. Date Prepared: October 2019. 2019/ADV/CAL/150H NEW TREATS HEARTBURN AND ACID REFLUX. ONE TABLET PER DAY. LASTS 24 HOURS. AVAILABLE IN PACKS OF 7s AND 14s. Marketed by CCF:22656 Date of preparation: (10-19) ABBREVIATED PRESCRIBING INFORMATION Product Name: Emazole Control 20 mg Gastro-Resistant Tablets Composition: Each tablet contains 20 mg esomeprazole (as magnesium dihydrate). Description: Light pink oval film coated tablet. Indication(s): Proton Pump Inhibitor (PPI): Short-term treatment of reflux symptoms (e.g. heartburn and acid regurgitation) in adults. Dosage: Swallow tablets whole with liquid, do not chew or crush. Disperse in half a glass of non-carbonated water if difficulty in swallowing. Stir until tablets disintegrate, drink liquid with pellets immediately or within 15 min, or administer through a gastric tube. Do not chew or crush pellets. Adults: The recommended dose is 20 mg esomeprazole (one tablet) per day. -

Explaining Corporate Success Abstract
Explaining corporate success: Britain’s best performing firms, 1949-1985 D.M. Higgins, S. Toms The York Management School Abstract The paper synthesises theories of competitive advantage in a dynamic framework to explain the determinants of corporate success. A model is presented linking the firm’s resource audit and dynamic capabilities with the rate of market growth. For the purposes of testing the model, an examination of the performance of all British quoted companies during the period is conducted with reference to achieved long run average rates of return on capital employed. The best performing firms are analysed in more detail and their strategies mapped according to the model criteria. Conclusions are then drawn on the possible strategies for achieving long run corporate success in terms of above average financial returns. Correspondence Steven Toms Joint Editor: Business History Professor of Accounting and Finance and Head of School The York Management School Sally Baldwin Buildings University of York York YO10 5DD Tel: (44) 1904-434122 Fax: (44) 1904-433 431 Email: [email protected] http://www.york.ac.uk/management/staff/StaffProfiles/SToms.htm 1 Explaining corporate success: Britain’s best performing firms, 1949-1985 1. Introduction Competitive advantage and more specifically sustained competitive advantage and its determinants, has become an important research topic in the business and management and industrial organisation literatures. Sustained competitive advantage (SCA) implies not just achieving superior returns, but achieving them over a protracted period of time. It is therefore surprising that the overwhelming majority of this literature has not explored the notion of sustained competitive advantage from a historical perspective. -

Glaxosmithkline Plc Annual Report for the Year Ended 31St December 2000
GlaxoSmithKline 01 GlaxoSmithKline plc Annual Report for the year ended 31st December 2000 Contents Report of the Directors 02 Financial summary 03 Joint statement by the Chairman and the Chief Executive Officer 05 Description of business 29 Corporate governance 37 Remuneration report 47 Operating and financial review and prospects 69 Financial statements 70 Directors’ statements of responsibility 71 Report by the auditors 72 Consolidated statement of profit and loss 72 Consolidated statement of total recognised gains and losses 74 Consolidated statement of cash flow 76 Consolidated balance sheet 76 Reconciliation of movements in equity shareholders’ funds 77 Company balance sheet 78 Notes to the financial statements 136 Group companies 142 Principal financial statements in US$ 144 Financial record 153 Investor information 154 Shareholder return 156 Taxation information for shareholders 157 Shareholder information 158 Share capital 160 Cross reference to Form 20-F 162 Glossary of terms The Annual Report was approved by the Board 163 Index of Directors on 22nd March 2001 and published on 12th April 2001. Contact details 02 GlaxoSmithKline Financial summary 2000 1999 Increase Business performance £m £m CER % £ % Sales 18,079 16,164 9 12 Trading profit 5,026 4,378 12 15 Profit before taxation 5,327 4,708 11 13 Earnings/Net income 3,697 3,222 13 15 Earnings per Ordinary Share 61.0p 52.7p 14 16 Total results Profit before taxation 6,029 4,236 Earnings/Net income 4,154 2,859 Earnings per Ordinary Share 68.5p 46.7p Business performance: results exclude merger items and restructuring costs; 1999 sales and trading profit exclude the Healthcare Services businesses which were disposed of in 1999. -

Paul M Mahan Matthews
17 April 2015 PAUL McMAHAN MATTHEWS [email protected] PRIMARY APPOINTMENTS July, 2014- Edmond and Lily Safra Professor of Translational Neuroscience and Therapeutics, Division of Brain Sciences, Department of Medicine, Imperial College London Secured endowment for, and first holder of, new Chair associated with the Divisional Head and posts (Edmond and Lily Safra Scholars) accompanying its foundation. April, 2012- Professor and Head, Division of Brain Sciences, Department of Medicine, Imperial College London Leading a new integration of over 70 academics all clinical and basic neurosciences, neuropsychopharmacology, neurology and mental health within a Division of 200 staff in the Faculty of Medicine with senior management responsibilities across the three academic hospital sites of Imperial College, London. Additional responsibilities as Deputy Director (2008- ) of the Wellcome Trust- GSK Translational Medicine Training Programme, Deputy Director (2011- ) of the BRC Institute for Translational Medicine and Therapeutics, Biomedical Research Centre (BRC) Theme Lead for Neuroscience, co-Director of the Neurotechnology Network and the EPSRC Centre for Doctoral Training in Neurology and Research Advisory Board for the Data Science Institute. July, 2012- February 2014 Vice-President for Integrative Medicines Development, Neurosciences and Medicine Development Lead, GlaxoSmithKline (joint appointment with Imperial College) Leading late phase development programme. Senior advisor to the Head of Neurosciences and China R and D with specific