Codeine Art. 31
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Aqualis ASA (A Public Limited Liability Company Organised Under the Laws of Norway) Org.No
Aqualis ASA (A public limited liability company organised under the laws of Norway) Org.no. 913 757 424 The information in this prospectus (the "Prospectus") relates to (i) a underwritten rights issue (the “Rights Issue”) by Aqualis ASA (“Aqualis” or the “Company”, and when taken together with its consolidated subsidiaries, the “Group” or the “Aqualis Group”) and the listing on Oslo Børs of up to 8,882,575 new shares in the Company with a par value of NOK 0.1 each (the “Rights Issue Shares”), issued for a subscription price of NOK 3.96 per share (the “Subscription Price”), (ii) listing of 4,375,000 new shares in Aqualis, each with a par value of NOK 0.1 each (the “Private Placement Shares”) and (iii) listing of 14,865,621 new shares in Aqualis, each with a par value of NOK 0.1 each (the “Consideration Shares”, and together with the Rights Issue shares and the Private Placement Shares the “New Shares”). Subscription Period for the Rights Issue: From 24 June 09:00 (CET) to 8 July 16:30 (CET) Trading in Subscription Rights: From 24 09:00 (CET) 4 July 16:30 (CET) The shareholders of the Company as at 11 June 2019 (and being registered as such in the Norwegian Central Securities Depository (the "VPS") on 13 June 2019 pursuant to the two days' settlement procedure (the "Record Date")) (the "Existing Shareholders"), will be granted subscription rights in the Rights Issue that, subject to applicable law, provide preferential rights to subscribe for and be allocated Rights Issue Shares at the Subscription Price (the "Subscription Rights"). -

Aqualis Offshore Holding ASA (A Public Limited Liability Company Organised Under the Laws of Norway) Org.No
Aqualis Offshore Holding ASA (A public limited liability company organised under the laws of Norway) Org.no. 913 757 424 Listing of 43,190,544 shares in Aqualis Offshore Holding ASA (the “Shares”) on the Oslo Stock Exchange (the “Listing”) The first day of listing of Aqualis Offshore Holding ASA (“New Aqualis” or the “Company”, together with its subsidiaries the “New Aqualis Group” or the “Group”) on the Oslo Stock Exchange is expected to be 13 August 2014 and trading in the shares will commence on the date of listing, under the trading symbol AQUA. Investing in the Shares involves a high degree of risk. See section 2 “Risk Factors” beginning on page 15. THIS PROSPECTUS SERVES AS A LISTING PROSPECTUS ONLY, AS REQUIRED BY NORWEGIAN LAW AND REGULATION. THIS PROSPECTUS DOES NOT CONSTITUE AN OFFER TO BUY, SUBSCRIBE OR SELL ANY OF THE SECURITIES DESCRIBED HEREIN, AND NO SECURITIES ARE BEING OFFERED OR SOLD PURSUANT TO IT. Manager: 12 August 2014 AQUALIS OFFSHORE HOLDING ASA IMPORTANT INFORMATION For the definitions of terms used throughout this Prospectus, see section 17 "Definitions and glossary of terms" of this Prospectus. This prospectus (the “Prospectus”) has been prepared to comply with the Norwegian Securities Trading Act of 29 June 2007 no. 75 (the “Norwegian Securities Trading Act”) and related secondary legislation, including the Commission Regulation (EC) no. 809/2004 implementing Directive 2003/71/EC of the European Parliament and of the Council of 4 November 2003 regarding information contained in prospectuses (the “Prospectus Directive”) as well as the format, incorporation by reference and publication of such prospectuses and dissemination of advertisements (hereafter “EC Regulation 809/2004”). -
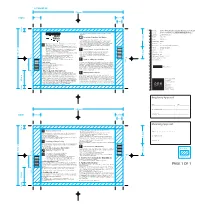
Solpadeine Max Tabs 03
File title: 9034_SOMT01240812v1_Solpadeine Max tabs 20s-30s PIL v3F • If you take a painkiller for headaches for more than 3 days it can make Item No.: 11000000010649 PROSP SOLPADEINA MAX TABLETAS UK 1212 them worse. Last edit: 22nd November 2013 Do not take Solpadeine Max Tablets: Brand: Solpadeine Tablets Variant: MAX Tablets • If you have ever had an allergic reaction to paracetamol, codeine, other opioid painkillers or to any of the other ingredients (listed in Section 6) Component: Leaflet • If you are taking other medicines containing paracetamol or codeine • For pain relief in children and adolescents (0-18 years of age) after removal • This medicine is for the short term treatment of acute moderate pain Market/s: UK of their tonsils or adenoids due to obstructive sleep apnoea syndrome when other painkillers have not worked. • If you know that you metabolise very rapidly codeine into morphine • You should only take this product for a maximum of 3 days at a time. If Dimensions: 150 x 100mm (Drw No. L613D005) • If you are breastfeeding. you need to take it for longer than 3 days you should see your doctor or pharmacist for advice. Printer: Santiago Gonzalez • This medicine contains codeine which can cause addiction if you take it Ask your doctor before you take this medicine: continuously for more than 3 days. This can give you withdrawal Substrate: Paper symptoms from the medicine when you stop taking it. • If you have liver or kidney disease, including alcoholic liver disease Tablets EAN Code: N/A • If you take this medicine for headaches for more than 3 days it can make • If you have bowel problems including blockage of your bowel them worse. -

Package Leaflet: Information for the User Trampalgin 37.5 Mg/325 Mg
Package leaflet: Information for the user Trampalgin 37.5 mg/325 mg tablets tramadol hydrochloride/paracetamol Read all of this leaflet carefully before you start taking this medicine because it contains important information for you. • Keep this leaflet. You may need to read it again. • If you have any further questions, ask your doctor or pharmacist or nurse. • This medicine has been prescribed for you only. Do not pass it on to others. It may harm them, even if their signs of illness are the same as yours. • If you get any side effects, talk to your doctor or pharmacist or nurse. This includes any possible side effects not listed in this leaflet. See section 4. What is in this leaflet: 1. What Trampalgin is and what it is used for 2. What you need to know before you take Trampalgin 3. How to take Trampalgin 4. Possible side effects 5. How to store Trampalgin 6. Contents of the pack and other information 1. What Trampalgin is and what it is used for Trampalgin is composed of two pain relieving active substances, tramadol and paracetamol. Trampalgin is used to treat moderate to severe pain when your doctor recommends that a combination of tramadol and paracetamol is needed. Trampalgin is intended for adults, adolescents and children over 12 years of age. 2. What you need to know before you take Trampalgin Do not take Trampalgin • if you are allergic (hypersensitive) to the active substances tramadol and paracetamol or any of the other ingredients of Trampalgin • in cases of acute poisoning with alcohol, sleeping pills, pain relievers or psychotropic drugs (that affect mood, emotions and state of mind). -

Glaxosmithkline Plc Annual Report for the Year Ended 31St December 2000
GlaxoSmithKline 01 GlaxoSmithKline plc Annual Report for the year ended 31st December 2000 Contents Report of the Directors 02 Financial summary 03 Joint statement by the Chairman and the Chief Executive Officer 05 Description of business 29 Corporate governance 37 Remuneration report 47 Operating and financial review and prospects 69 Financial statements 70 Directors’ statements of responsibility 71 Report by the auditors 72 Consolidated statement of profit and loss 72 Consolidated statement of total recognised gains and losses 74 Consolidated statement of cash flow 76 Consolidated balance sheet 76 Reconciliation of movements in equity shareholders’ funds 77 Company balance sheet 78 Notes to the financial statements 136 Group companies 142 Principal financial statements in US$ 144 Financial record 153 Investor information 154 Shareholder return 156 Taxation information for shareholders 157 Shareholder information 158 Share capital 160 Cross reference to Form 20-F 162 Glossary of terms The Annual Report was approved by the Board 163 Index of Directors on 22nd March 2001 and published on 12th April 2001. Contact details 02 GlaxoSmithKline Financial summary 2000 1999 Increase Business performance £m £m CER % £ % Sales 18,079 16,164 9 12 Trading profit 5,026 4,378 12 15 Profit before taxation 5,327 4,708 11 13 Earnings/Net income 3,697 3,222 13 15 Earnings per Ordinary Share 61.0p 52.7p 14 16 Total results Profit before taxation 6,029 4,236 Earnings/Net income 4,154 2,859 Earnings per Ordinary Share 68.5p 46.7p Business performance: results exclude merger items and restructuring costs; 1999 sales and trading profit exclude the Healthcare Services businesses which were disposed of in 1999. -

Malta Medicines List April 08
Defined Daily Doses Pharmacological Dispensing Active Ingredients Trade Name Dosage strength Dosage form ATC Code Comments (WHO) Classification Class Glucobay 50 50mg Alpha Glucosidase Inhibitor - Blood Acarbose Tablet 300mg A10BF01 PoM Glucose Lowering Glucobay 100 100mg Medicine Rantudil® Forte 60mg Capsule hard Anti-inflammatory and Acemetacine 0.12g anti rheumatic, non M01AB11 PoM steroidal Rantudil® Retard 90mg Slow release capsule Carbonic Anhydrase Inhibitor - Acetazolamide Diamox 250mg Tablet 750mg S01EC01 PoM Antiglaucoma Preparation Parasympatho- Powder and solvent for solution for mimetic - Acetylcholine Chloride Miovisin® 10mg/ml Refer to PIL S01EB09 PoM eye irrigation Antiglaucoma Preparation Acetylcysteine 200mg/ml Concentrate for solution for Acetylcysteine 200mg/ml Refer to PIL Antidote PoM Injection injection V03AB23 Zovirax™ Suspension 200mg/5ml Oral suspension Aciclovir Medovir 200 200mg Tablet Virucid 200 Zovirax® 200mg Dispersible film-coated tablets 4g Antiviral J05AB01 PoM Zovirax® 800mg Aciclovir Medovir 800 800mg Tablet Aciclovir Virucid 800 Virucid 400 400mg Tablet Aciclovir Merck 250mg Powder for solution for inj Immunovir® Zovirax® Cream PoM PoM Numark Cold Sore Cream 5% w/w (5g/100g)Cream Refer to PIL Antiviral D06BB03 Vitasorb Cold Sore OTC Cream Medovir PoM Neotigason® 10mg Acitretin Capsule 35mg Retinoid - Antipsoriatic D05BB02 PoM Neotigason® 25mg Acrivastine Benadryl® Allergy Relief 8mg Capsule 24mg Antihistamine R06AX18 OTC Carbomix 81.3%w/w Granules for oral suspension Antidiarrhoeal and Activated Charcoal -

Let Food (Supplements) Be Thy Medicine
4-Feb-19 7:40 AM Italian Equity Research PHARMANUTRA BUY Sector: Pharmaceutical New Coverage TP 21.50 Let Food (Supplements) Be Thy Medicine New Coverage Target price upside: +25% We initiate coverage on Pharmanutra with a BUY rating and a Target Price of EUR21.50 per FY18E FY19E share, providing 25% upside to the current share price. The Group is an undisputed leader in Change in EPS est. iron food supplements market (Sideral® has gained a 50% market share in Italy), and a 0.0% 0.0% credible challenger in the topical anti-inflammatory market (Cetilar® is quickly gaining market share). Thanks to an Intellectual Property (IP) production and management strategy Ticker (BBG, Reut) PHN IM PHN MI incomparable within the dietary supplements and medical devices industry, Pharmanutra Share price Ord. (Eu) 17.3 has created long-lasting competitive advantages and high barriers to protect its assets. The N. of Ord. shares (mn) 9.7 Group owns 8 proprietary raw materials, 7 patents based on pure technological innovation, Total N. of shares (mn) 9.7 25 registered brands, and has 79 completed clinical trials. We expect the impressive growth Market cap (Eu mn) 167 track record to continue in the medium term (we forecast a high-teens revenue and profit Total Market Cap (EU mn) 167 CAGR in 2017-20) driven by: the development of new formulations/applications for Sideral® Free Float Ord. (%) 29% and Cetilar® families of products, the launch of brand-new products (Sucrosomial Free Float Ord. (Eu mn) 48 magnesium®), and a wider penetration of foreign markets (the Group is constantly entering Daily AVG liquidity Ord. -
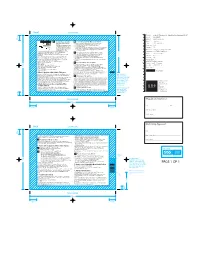
Solpadeine Max Soluble Tabs 03
FRONT LEADING EDGE File title: 9034_SOLPM00890812v1_Solpadeine Max Soluble tabs PIL v5F Item No.: 40L564D(OPI) Last edit: 22nd November 2013 8.2mm This medicine is for the short term Brand: Solpadeine treatment of acute moderate pain Do not take Solpadeine Max Soluble Tablets: when other painkillers have not If you have ever had an allergic reaction Variant: MAX soluble tablets worked. You should only take this product for taking other medicines containing paracetamol. Component: Leaflet a maximum of 3 days at a time. If under 12 years: you need to take it for longer than 3 For pain relief in children and adolescents (0-18 years of age) after removal Market/s: UK of their tonsils or adenoids due to obstructive sleep apnoea syndrome days you should see your doctor Dimensions: 150 x 105mm (Drw No. OTC.LT.008) or pharmacist for advice. If you know that you metabolise very rapidly codeine into morphine If you are breastfeeding. This medicine contains codeine, which can cause addition if you take it 64.2mm Copy position: 1 OUTSIDE, 5 INSIDE print continuously for more than 3 days. This can give you withdrawal Ask your doctor before you take this medicine: symptoms from the medicine when you stop taking it. Printer: Chesapeake St Pierre If you take this medicine for headaches for more than 3 days, it can make liver kidney disease alcoholic liver disease them worse. bowel problems Substrate: Paper Please read right through this leaflet before you start using this medicine. operation to remove your gall bladder intolerance to EAN Code: N/A some sugars controlled sodium diet. -

Spec Pharma M&A Transaction Multiples
SECTOR REPORT FOURTH QUARTER 2016 Disclaimer All information set forth in this report (the “Overview”) has been synthesized by Bourne Capital Partners, L.L.C. (“BP”) or was obtained from publicly available sources. BP makes no express or implied representation or warranty as to the accuracy or completeness of the information contained herein. BP expressly disclaims any and all liability that may be based on all information set forth in the Overview, errors therein, or omissions therefrom. This Overview includes certain statements, estimates and projections provided by BP with respect to anticipated future performance. Such statements, estimates and projections reflect various assumptions made by BP concerning anticipated results, which reflect significant subjective judgments made by BP and as a result, may or may not prove to be correct. There can be no assurance that such projected results are attainable or will be realized. No express or implied representations or warranties are made as to the accuracy of such statements, estimates or projections. In furnishing the Overview, BP does not undertake any obligation to provide the recipient with access to any additional information, to correct any inaccuracies that may become apparent or to update or otherwise revise this Overview. This Overview is not an offer to sell or a solicitation of an offer to purchase securities or to engage in any other transaction. BP is a North Carolina (USA) limited liability company doing business as Bourne Partners with divisions in Healthcare Merchant Banking, Alternative Assets, Management Consulting and Investment Banking. Investment Banking services are offered by Bourne Partners Securities, LLC, a registered broker dealer, Member FINRA and SIPC. -

Oesophageal Stricture Associated with Emepronium Bromide Therapy 1
Postgrad Med J: first published as 10.1136/pgmj.58.675.43 on 1 January 1982. Downloaded from Postgraduate Aledical Journal (January 1982) 58, 43-44 Oesophageal stricture associated with emepronium bromide therapy 1. W. FELLOWS A. L. OGILVIE M.B., M.R.C.P. M.B., M.R.C.P. M. ATKINSON M.D., F.R.C.P. University Hospital, Queen's Medical Centre, Nottingham Summary Barium swallow showed a small fixed hiatus Emepronium bromide, a drug used to control urinary hernia with a stricture above it and an ulcer crater frequency, has been reported as causing oesophageal therein. ulceration but not stricture formation. This paper Endoscopy showed a 5-mm diameter benign presents 3 cases in which the use of emepronium stricture at 33 cm from the alveolar margin; the bromide preceded development of an oesophageal stricture was 1 cm long and was ulcerated. Below it stricture and suggests that the drug played a causative was a hiatus hernia. The stricture was dilated, the role. emepronium stopped and she was given cimetidine and Asilone gel. One further dilatation was needed Case reports 4 months later but she has swallowed well for the Case I last 6 months. copyright. A 50-year-old woman, with a 6-year history of multiple sclerosis causing spastic paraparesis of the Case 3 lower limbs, presented with a 2-month history of A 74-year-old woman presented with progressive dysphagia for solids, retrosternal pain and 12-7 kg dysphagia for solids for a few weeks and mild weight loss. She was taking baclofen, carbamazepine, heartburn. -

Annual Report 2008 Find out More About GSK Online…
Do more, feel better, live longer Grow Deliver Simplify Annual Report 2008 Find out more about GSK online… www.gsk.com Website GlaxoSmithKline’s website www.gsk.com gives additional information on the Group. Information made available on the website does not constitute part of this Annual Report. Notice regarding limitations on Director liability under English Law Under the UK Companies Act 2006, a safe harbour limits the liability of Directors in respect of statements in and omissions from the Report of the Directors contained on pages 12 to 98. Under English law the Directors would be liable to the company (but not to any third party) if the Report of the Directors contains errors as a result of recklessness or knowing misstatement or dishonest concealment of a material fact, but would not otherwise be liable. Report of the Directors Pages 12 to 98 inclusive consist of a Report of the Directors that has been drawn up and presented in accordance with and in reliance upon English company law and the liabilities of the Directors in connection with that report shall be subject to the limitations and restrictions provided by such law. Cautionary statement regarding forward-looking statements The Group’s reports filed with or furnished to the US Securities and Exchange Commission (SEC), including this document and written information released, or oral statements made, to the public in the future by or on behalf of the Group, may contain forward-looking statements. Forward-looking statements give the Group’s current expectations or forecasts of future events. A shareholder can identify these statements by the fact that they do not relate strictly to historical or current facts. -

Self Help Gastrointestinal System Irritable Bowel Syndrome (IBS)
Self Help This guide is to help you see which conditions you can safely try treating yourself or with the advise of your pharmacist. For information on all conditions listed below (and many more) see www.NHS.uk - HEALTH A-Z Prices: The guide below gives an indication of cost. At the time of writing (July 2019) the cost of a single medication on prescription in England was £9 (see www.NHS.UK for up to date charges) £ ££ £££ 50p - £2 £2 - £6 £6-£10 Gastrointestinal System Dyspepsia or acid reflux (Indigestion or heart burn) • Discomfort in the upper abdomen or chest often after eating. Associated with feeling sick, full or bloated. Bringing up food or bitter tasting liquids. See GP if : keep getting indigestion • are in bad pain • are 55 or older • have lost a lot of weight without meaning to • have difficulty swallowing (dysphagia) • keep vomiting • have iron deficiency anaemia • feel like you have a lump in your stomach • have bloody vomit or poo These symptoms can be a sign of something more serious. Name of Medicine Cost Gaviscon® Advance Liquid £££ Gaviscon ®Advance Tablets ££ Ranitidine 75mg Tablets £ Irritable Bowel Syndrome (IBS) A common condition affecting the digestive system causing, stomach cramps, bloating, diarrhea and/or constipation. Often a lifelong problem. For more information see https://www.bda.uk.com/foodfacts/IBSfoodfacts.pdf See GP if there is bleeding when passing stool, a family history of Inflammatory bowel disease or coeliacs, or a family history of ovarian cancer, or if the condition is persistent and intrusive. Produced by: The Medicine Box and The University Medical Centre Updated: August 2019 If you are over 60 and develop new symptoms like this see your GP.