Lynx Canadensis)
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

(Muhv 4) Strains: a Role for Immunomodulatory Proteins Encoded by the Left (5’-)End of the Genome
Cent. Eur. J. Biol. • 3(1) • 2008 • 19-30 DOI: 10.2478/s11535-008-0002-0 Central European Journal of Biology Comparison of pathogenic properties of the murid gammaherpesvirus (MuHV 4) strains: a role for immunomodulatory proteins encoded by the left (5’-)end of the genome Review Article Jela Mistríková1,2, Július Rajčáni2* 1 Department of Microbiology and Virology, Faculty of Microbiology and Natural Sciences, Comenius University, 84215 Bratislava, Slovakia 2 Institute of Virology, Slovak Academy of Sciences, 84505 Bratislava, Slovakia Received 13 September 2007; Accepted 4 January 2008 Abstract: The murid herpesvirus 4 (MuHV 4) species encompasses 7 isolates, out of which at least two (MHV-68, MHV-72) became in vitro propagated laboratory strains. Following intranasal inoculation, MuHV 4 induces an acute infectious mononucleosis-like syndrome with elevated levels of peripheral blood leukocytes, shifts in the relative proportion of lymphocytes along with the appearance of atypi- cal mononuclear cells. At least two isolates exhibited spontaneous deletions at the left hand (5´-end) of their genome, resulting in the absence of M1, M2, M3 genes (strain MHV-72) and also of the M4 gene (strain MHV-76). Based on DNA sequence amplifications only, another two isolates (MHV-Šum and MHV-60) were shown to possess similar deletions of varying length. During latency (until 24 months post-infection), the mice infected with any MuHV 4 isolate (except MHV-76) developed lymphoproliferative disorders. The lack of tumor formation in MHV-76 infected mice was associated with persistent virus production at late post-infection intervals. In addition to careful analysis of spontaneously occurring 5´-end genome defects, our knowledge of the function of 5´-end genes relies on the behaviour of mutants with corresponding deletions and/or insertions. -

Discovery of a Novel Bat Gammaherpesvirus
COMMENTARY Host-Microbe Biology crossmark Discovery of a Novel Bat Gammaherpesvirus Kurtis M. Host,a,b Blossom Damaniaa,b Lineberger Comprehensive Cancer Centera and Department of Microbiology and Immunology,b University of North Carolina at Chapel Hill, Chapel Hill, North Carolina, USA ABSTRACT Zoonosis is the leading cause of emerging infectious diseases. In a re- cent article, R. S. Shabman et al. (mSphere 1[1]:e00070-15, 2016, 10.1128/ Published 17 February 2016 mSphere.00070-15) report the identification of a novel gammaherpesvirus in a cell Citation Host KM, Damania B. 2016. Discovery of a novel bat gammaherpesvirus. mSphere line derived from the microbat Myotis velifer incautus. This is the first report on a 1(1):e00016-16. doi:10.1128/mSphere.00016- replicating, infectious gammaherpesvirus from bats. The new virus is named bat 16. gammaherpesvirus 8 (BGHV8), also known as Myotis gammaherpesvirus 8, and is Copyright © 2016 Host and Damania. This is able to infect multiple cell lines, including those of human origin. Using next- an open-access article distributed under the terms of the Creative Commons Attribution 4.0 generation sequencing technology, the authors constructed a full-length annotated International license. genomic map of BGHV8. Phylogenetic analysis of several genes from BGHV8 re- Address correspondence to Blossom Damania, vealed similarity to several mammalian gammaherpesviruses, including Kaposi’s [email protected]. sarcoma-associated herpesvirus (KSHV). The views expressed in this Commentary do not necessarily reflect the views of the journal or of ASM. KEYWORDS: Myotis velifer incautus, bat, BGHV8, gammaherpesvirus, Myotis Discovery of a novel bat gammaherpesvirus 8 gammaherpesvirus merging infectious diseases (EID), a significant financial burden and public health Ethreat, are on the rise (1). -

Development of In-House Taqman Qpcr Assay to Detect Equine Herpesvirus-2 in Al-Qadisiyah City ﻟﺛﺎﻧﻲ ا ﻓﺎﯾرو
Iraqi Journal of Veterinary Sciences, Vol. 34, No. 2, 2020 (365-371) Development of in-house Taqman qPCR assay to detect equine herpesvirus-2 in Al-Qadisiyah city M.H. Al-Saadi Department of Internal and Preventive Medicine, College of Veterinary Medicine, University of Al-Qadisiyah, Al-Qadisiyah, Iraq, Email: [email protected] (Received September 6, 2019; Accepted October 1, 2019; Available online July 23, 2020) Abstract EHV-2 is distributed in horses globally. It is clustered within gamma-herpesvirus subfamily and percavirus genus. EHV-2 infection has two phases: latent and lytic. In the later, EHV-2 mainly associated with respiratory and genital symptoms. However, in the quiescent phase of infection, EHV-2 stay dormant in the host till viral reactivation. Our previous study has showed that EHV-2 can be harboured by equine tendons, suggesting that leukocytes possibly carrying EHV-2 for the systemic dissemination. So far, numerous PCR protocols have been performed targeting the gB gene. However, this gene is heterogenic. Therefore, there is a need to develop a quantitative diagnostic approach to detect the quiescent EHV-2 strains. To do this, Taqman qPCR assay was developed to quantify the virus. This was performed by targeting a highly conserved gene known as DNA polymerase (DPOL) gene using constructed plasmid as a standard curve calibrator. The obtained results showed an infection frequency of 33% in which the EHV-2 load reached 6647 copies/100 ng DNA whereas the minimum load revealed as 2 copies/100 ng DNA. The median quantification was found as 141 copies/ 100 ng DNA. -

Development and Application of a Quantitative Pcr Assay to Study the Pathogenicity of Equine Herpesvirus 5
DEVELOPMENT AND APPLICATION OF A QUANTITATIVE PCR ASSAY TO STUDY THE PATHOGENICITY OF EQUINE HERPESVIRUS 5 By Lila Marek Zarski A THESIS Submitted to Michigan State University in partial fulfillment of the requirements for the degree of Comparative Medicine and Integrative Biology – Master of Science 2016 ABSTRACT DEVELOPMENT AND APPLICATION OF A QUANTITATIVE PCR ASSAY TO STUDY THE PATHOGENICITY OF EQUINE HERPESVIRUS 5 By Lila Marek Zarski Equine herpesvirus 5 (EHV-5) infection is associated with pulmonary fibrosis in horses, but further studies on EHV-5 persistence in equine cells are needed to fully understand viral and host contributions to disease pathogenesis. We developed a quantitative PCR (qPCR) assay to measure EHV-5 viral copy number in equine cell culture, blood lymphocytes, and nasal swabs of horses. The PCR primers and a probe were designed to target gene E11 of the EHV-5 genome. Specificity was verified by testing multiple isolates of EHV-5, as well as DNA from other equine herpesviruses. Four-week old, fully differentiated (mature) and newly seeded (immature) primary equine respiratory epithelial cell (ERECs) cultures were inoculated with EHV-5 and the cells and supernatants collected daily for 12-14 days. Blood lymphocytes and nasal swabs were collected from horses experimentally infected with EHV-1. The qPCR assay detected EHV-5 at concentrations around 104 intracellular genomes per cell culture in experimentally inoculated mature ERECs, and these values remained stable throughout 12 days. Intracellular EHV-5 copies detected in the immature cultures increased over 14 days and reached levels greater than 106 genomes per culture. EHV-5 was detected in the lymphocytes of 97% of horses and in the nasal swabs of 88% of horses both pre and post EHV-1 infection. -

Radio Essentials 2012
Artist Song Series Issue Track 44 When Your Heart Stops BeatingHitz Radio Issue 81 14 112 Dance With Me Hitz Radio Issue 19 12 112 Peaches & Cream Hitz Radio Issue 13 11 311 Don't Tread On Me Hitz Radio Issue 64 8 311 Love Song Hitz Radio Issue 48 5 - Happy Birthday To You Radio Essential IssueSeries 40 Disc 40 21 - Wedding Processional Radio Essential IssueSeries 40 Disc 40 22 - Wedding Recessional Radio Essential IssueSeries 40 Disc 40 23 10 Years Beautiful Hitz Radio Issue 99 6 10 Years Burnout Modern Rock RadioJul-18 10 10 Years Wasteland Hitz Radio Issue 68 4 10,000 Maniacs Because The Night Radio Essential IssueSeries 44 Disc 44 4 1975, The Chocolate Modern Rock RadioDec-13 12 1975, The Girls Mainstream RadioNov-14 8 1975, The Give Yourself A Try Modern Rock RadioSep-18 20 1975, The Love It If We Made It Modern Rock RadioJan-19 16 1975, The Love Me Modern Rock RadioJan-16 10 1975, The Sex Modern Rock RadioMar-14 18 1975, The Somebody Else Modern Rock RadioOct-16 21 1975, The The City Modern Rock RadioFeb-14 12 1975, The The Sound Modern Rock RadioJun-16 10 2 Pac Feat. Dr. Dre California Love Radio Essential IssueSeries 22 Disc 22 4 2 Pistols She Got It Hitz Radio Issue 96 16 2 Unlimited Get Ready For This Radio Essential IssueSeries 23 Disc 23 3 2 Unlimited Twilight Zone Radio Essential IssueSeries 22 Disc 22 16 21 Savage Feat. J. Cole a lot Mainstream RadioMay-19 11 3 Deep Can't Get Over You Hitz Radio Issue 16 6 3 Doors Down Away From The Sun Hitz Radio Issue 46 6 3 Doors Down Be Like That Hitz Radio Issue 16 2 3 Doors Down Behind Those Eyes Hitz Radio Issue 62 16 3 Doors Down Duck And Run Hitz Radio Issue 12 15 3 Doors Down Here Without You Hitz Radio Issue 41 14 3 Doors Down In The Dark Modern Rock RadioMar-16 10 3 Doors Down It's Not My Time Hitz Radio Issue 95 3 3 Doors Down Kryptonite Hitz Radio Issue 3 9 3 Doors Down Let Me Go Hitz Radio Issue 57 15 3 Doors Down One Light Modern Rock RadioJan-13 6 3 Doors Down When I'm Gone Hitz Radio Issue 31 2 3 Doors Down Feat. -

Prevalence and Risk Factors for Felis Catus Gammaherpesvirus 1
Veterinary Microbiology 238 (2019) 108426 Contents lists available at ScienceDirect Veterinary Microbiology journal homepage: www.elsevier.com/locate/vetmic Prevalence and risk factors for Felis catus gammaherpesvirus 1 detection in T domestic cats in Italy Francesca Caringellaa, Costantina Desarioa, Eleonora Lorussoa, Ivana Pallantea, Tommaso Furlanellob, Gianvito Lanavea, Gabriella Eliaa, Vito Martellaa, Roberta Iattaa, ⁎ Vanessa R. Barrsc, Julia Beattyc, Canio Buonavogliaa, Nicola Decaroa, a Department of Veterinary Medicine, University of Bari, Valenzano, Bari, Italy b San Marco Veterinary Clinic and Laboratory, Veggiano, Padova, Italy c Faculty of Science, Sydney School of Veterinary Science, The University of Sydney, Sydney, NSW, 2006, Australia ARTICLE INFO ABSTRACT Keywords: Felis catus gammaherpesvirus 1 (FcaGHV1), a novel gammaherpesvirus of domestic cats identified in 2014, has Cats been detected in different countries demonstrating a worldwide distribution. The aim of this study wastoes- Gammaherpesvirus tablish the prevalence of FcaGHV1 in Italy using a molecular epidemiological approach. FcaGHV1 DNA was Molecular survey detected with virus-specific real-time PCR in ≃1% of 2659 feline blood samples tested. Analysis of risk factors Risk factors showed that being male and coinfection with feline immunodeficiency virus (FIV) increase the likelihood of FcaGHV1 detection. One-third of FcaGHV1-positive cats also tested positive for FIV provirus, whereas coin- fections with feline panleukopenia virus were not demonstrated. Further studies are necessary to confirm the risk factors for FcaGHV1 detection and the pathobiology of the virus. 1. Introduction McLuckie et al., 2016a,b; Kurissio et al., 2018; Troyer et al., 2014), with detection rates between 9.6 and 23.6%. Herpesviridae are double-stranded DNA viruses (130-220 kbp) that Risk factors for FcaGHV1 infection are reported to be age, sex, comprise three subfamilies (Alpha-, Beta-, and Gammaherpesvirinae) on health status and coinfections with other microorganisms. -

The Critical Role of Genome Maintenance Proteins in Immune Evasion During Gammaherpesvirus Latency
fmicb-09-03315 January 4, 2019 Time: 17:18 # 1 REVIEW published: 09 January 2019 doi: 10.3389/fmicb.2018.03315 The Critical Role of Genome Maintenance Proteins in Immune Evasion During Gammaherpesvirus Latency Océane Sorel1,2 and Benjamin G. Dewals1* 1 Immunology-Vaccinology, Department of Infectious and Parasitic Diseases, Faculty of Veterinary Medicine-FARAH, University of Liège, Liège, Belgium, 2 Department of Molecular Biology and Biochemistry, University of California, Irvine, Irvine, CA, United States Gammaherpesviruses are important pathogens that establish latent infection in their natural host for lifelong persistence. During latency, the viral genome persists in the nucleus of infected cells as a circular episomal element while the viral gene expression program is restricted to non-coding RNAs and a few latency proteins. Among these, the genome maintenance protein (GMP) is part of the small subset of genes expressed in latently infected cells. Despite sharing little peptidic sequence similarity, gammaherpesvirus GMPs have conserved functions playing essential roles in latent Edited by: Michael Nevels, infection. Among these functions, GMPs have acquired an intriguing capacity to evade University of St Andrews, the cytotoxic T cell response through self-limitation of MHC class I-restricted antigen United Kingdom presentation, further ensuring virus persistence in the infected host. In this review, we Reviewed by: Neil Blake, provide an updated overview of the main functions of gammaherpesvirus GMPs during University of Liverpool, latency with an emphasis on their immune evasion properties. United Kingdom James Craig Forrest, Keywords: herpesvirus, viral latency, genome maintenance protein, immune evasion, antigen presentation, viral University of Arkansas for Medical proteins Sciences, United States *Correspondence: Benjamin G. -

Layout Final 22 09 05.Qxp
Mercado Brasileiro de Música Brazilian Music Market 2004 Mercado Brasileiro de Música Brazilian Music Market 2004 2005. ABPD - Associação Brasileira dos Produtores de Discos Qualquer parte desta obra poderá ser reproduzida, desde que citada a fonte. Any part of this report can be reproduced provided the source is indicated. Este documento foi elaborado por uma equipe cujos nomes encontram-se relacionados na folha de créditos. This document was prepared by a team and their names are listed in the acknowledgements. Ficha Catalográfica ABPD. Publicação Anual do Mercado Fonográfico ABPD 2004 Rio de Janeiro, 2005. 82 p. TÍTULO Catalogue Card ABPD - Annual Publication of the Recording Market 2004 Rio de Janeiro, 2005. 82 p. TITLE ABPD SEDE - HEADQUARTERS Associação Brasileira dos Produtores de Discos + Rua Marquês de São Vicente, 99 - 1º andar Gávea 22451-041 Rio de Janeiro RJ Brasil Brazilian Asssociation of Record Producers ( (+ 55 21) 2512-9908 4 (+ 55 21) 2259-4145 , [email protected] : http://www.abpd.org.br 02 Apresentação Foreword Pelo quinto ano consecutivo a ABPD - Associação Brasileira dos Produtores de Discos apre- The Brazilian Association of Record producers - ABPD is glad senta seu relatório anual sobre o mercado fonográfi- to publish its 5th annual report on the Brazilian music market. co brasileiro. The publication "Brazilian Music Market" contains relevant A publicação Mercado Brasileiro de Música information on the Brazilian and global phonographic markets for apresenta as principais informações sobre o mercado 2004. This year's edition also includes statistics, information on the fonográfico brasileiro e o mundial em 2004. Além de inserir nesta edição também, entre outra infor- fight against piracy, market research, consumer profiles in Brazil as mações: dados estatísticos do setor, incluindo o com- well as information on trade body ABPD company members. -

Evidence to Support Safe Return to Clinical Practice by Oral Health Professionals in Canada During the COVID-19 Pandemic: a Repo
Evidence to support safe return to clinical practice by oral health professionals in Canada during the COVID-19 pandemic: A report prepared for the Office of the Chief Dental Officer of Canada. November 2020 update This evidence synthesis was prepared for the Office of the Chief Dental Officer, based on a comprehensive review under contract by the following: Paul Allison, Faculty of Dentistry, McGill University Raphael Freitas de Souza, Faculty of Dentistry, McGill University Lilian Aboud, Faculty of Dentistry, McGill University Martin Morris, Library, McGill University November 30th, 2020 1 Contents Page Introduction 3 Project goal and specific objectives 3 Methods used to identify and include relevant literature 4 Report structure 5 Summary of update report 5 Report results a) Which patients are at greater risk of the consequences of COVID-19 and so 7 consideration should be given to delaying elective in-person oral health care? b) What are the signs and symptoms of COVID-19 that oral health professionals 9 should screen for prior to providing in-person health care? c) What evidence exists to support patient scheduling, waiting and other non- treatment management measures for in-person oral health care? 10 d) What evidence exists to support the use of various forms of personal protective equipment (PPE) while providing in-person oral health care? 13 e) What evidence exists to support the decontamination and re-use of PPE? 15 f) What evidence exists concerning the provision of aerosol-generating 16 procedures (AGP) as part of in-person -

Diversification of Mammalian Deltaviruses by Host Shifting
Diversification of mammalian deltaviruses by host shifting Laura M. Bergnera,b,1, Richard J. Ortonb, Alice Broosb, Carlos Telloc,d, Daniel J. Beckere, Jorge E. Carreraf,g, Arvind H. Patelb, Roman Bieka, and Daniel G. Streickera,b,1 aInstitute of Biodiversity, Animal Health and Comparative Medicine, College of Medical, Veterinary and Life Sciences, University of Glasgow, Glasgow G12 8QQ, Scotland; bMedical Research Center–University of Glasgow Centre for Virus Research, Glasgow G61 1QH, Scotland; cAssociation for the Conservation and Development of Natural Resources, 15037 Lima, Perú; dYunkawasi, 15049 Lima, Perú; eDepartment of Biology, University of Oklahoma, Norman, OK 73019; fDepartamento de Mastozoología, Museo de Historia Natural, Universidad Nacional Mayor de San Marcos, Lima 15081, Perú; and gPrograma de Conservación de Murciélagos de Perú, Piura 20001, Perú Edited by Paul E. Turner, Yale University, New Haven, CT, and approved November 25, 2020 (received for review September 22, 2020) Hepatitis delta virus (HDV) is an unusual RNA agent that replicates satellites either cospeciated with their hosts over ancient time- using host machinery but exploits hepatitis B virus (HBV) to scales or possess an unrecognized capacity for host shifting, mobilize its spread within and between hosts. In doing so, HDV which would imply their potential to emerge in novel species. enhances the virulence of HBV. How this seemingly improbable The latter scenario has been presumed unlikely since either both hyperparasitic lifestyle emerged is unknown, but it underpins the satellite and helper would need to be compatible with the novel likelihood that HDV and related deltaviruses may alter other host or deltaviruses would need to simultaneously switch host host–virus interactions. -
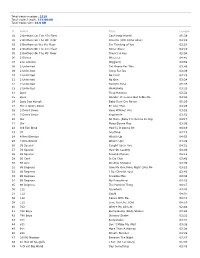
Total Tracks Number: 2218 Total Tracks Length: 151:00:00 Total Tracks Size: 16.0 GB
Total tracks number: 2218 Total tracks length: 151:00:00 Total tracks size: 16.0 GB # Artist Title Length 01 2 Brothers On The 4Th Floor Can't Help Myself 05:39 02 2 Brothers On The 4th Floor Dreams (Will Come Alive) 04:19 03 2 Brothers on the 4th Floor I'm Thinking of You 03:24 04 2 Brothers On The 4Th Floor Never Alone 04:10 05 2 Brothers On The 4th Floor There's A Key 03:54 06 2 Eivissa Oh La La 04:41 07 2 In a Room Wiggle It 03:59 08 2 Unlimited Get Ready For This 03:40 09 2 Unlimited Jump For Joy 03:39 10 2 Unlimited No Limit 03:28 11 2 Unlimited No One 03:24 12 2 Unlimited Twilight Zone 05:36 13 2 Unlimited Workaholic 03:33 14 2pac Thug Mansion 03:32 15 2pac Wonder If Heaven Got A Ghetto 04:34 16 2pac Daz Kurupt Baby Dont Cry Remix 05:20 17 Three Doors Down Be Like That 04:25 18 3 Doors Down Here Without You 03:53 19 3 Doors Down Kryptonite 03:52 20 3lw No More (Baby I'm Gonna Do Rig 04:17 21 3lw Playa Gonna Play 03:06 22 3rd Eye Blind How Is It Gonna Be 04:10 23 3T Anything 04:15 24 4 Non Blondes What's Up 04:55 25 4 Non Blonds What's Up? 04:09 26 38 Special Caught Up In You 04:25 27 38 Special Hold On Loosely 04:40 28 38 Special Second Chance 04:12 29 50 Cent In Da Club 03:42 30 50 cent Window Shopper 03:09 31 98 Degrees Give Me One More Night (Una No 03:23 32 98 Degrees I Do (Cherish You) 03:43 33 98 Degrees Invisible Man 04:38 34 98 Degrees My Everything 04:28 35 98 Degrees The Hardest Thing 04:27 36 112 Anywhere 04:03 37 112 Cupid 04:07 38 112 Dance With Me 03:41 39 112 Love You Like I Did 04:16 40 702 Where My Girls At 02:44 -

Medizinische Hochschule Hannover Detection of Murine Herpesvirus 68
Medizinische Hochschule Hannover Institut für Virologie/ Helmholtz Zentrum für Infektionsforschung Detection of murine herpesvirus 68 by the innate immune system and studies on Mus musculus rhadinovirus 1 in its natural host INAUGURALDISSERTATION zur Erlangung des Grades einer Doktorin der Naturwissenschaften - Doctor rerum naturalium - (Dr. rer. nat.) vorgelegt von Sripriya Murthy aus Bangalore Hannover 2016 Angenommen vom Senat der Medizinischen Hochschule Hannover am 16.09.2016 Gedruckt mit Genehmigung der Medizinischen Hochschule Hannover Präsident: Prof. Dr. med. Christopher Baum Betreuer: Prof. Dr. rer. nat. Melanie M. Brinkmann Kobetreuer: Prof. André Bleich, PhD 1. Gutachter: Prof. Dr. rer. nat. Melanie M. Brinkmann 2. Gutachter: Prof. André Bleich, PhD 3. Gutachter: Prof. Dr. rer. nat. Beate Sodeik Tag der mündlichen Prüfung vor der Prüfungskomission: 16.09.2016 Prof. Dr. rer. nat. Christine Falk Prof. Dr. rer. nat. Melanie M. Brinkmann Prof. André Bleich, PhD Prof. Dr. rer. nat. Beate Sodeik Table of contents 1 Introduction ............................................................................................................ 1 1.1 Herpesviridae .................................................................................................... 1 1.1.1 Betaherpesvirinae ......................................................................................... 2 1.1.2 Gammaherpesvirinae .................................................................................... 4 1.2 Host defense ....................................................................................................