Insect Sting Allergy David F
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Download WAO White Book on Allergy
WORLD ALLERGY ORGANIZATION WAWAOO WhiteWhite BookBook onon AllergyAllergy WAO White Book on Allergy World Allergy Organization (WAO) White Book on Allergy Copyright 2011 World Allergy Organization WAO White Book on Allergy Editors Prof. Ruby Pawankar, MD, PhD Prof. Giorgio Walter Canonica, MD WAO President Elect (2010-2011) WAO Past President (2010-2011) Allergy and Rhinology Allergy & Respiratory Diseases Nippon Medical School Department of Internal Medicine 1-1-5 Sendagi, Bunkyo-ku University of Genoa Tokyo 113-8603 Padiglione Maragliano, Largo Rosanna Benzi 10 JAPAN 1-16132 Genoa ITALY Prof. Stephen T. Holgate, BSc, MD, DSc, FMed Sci Prof. Richard F. Lockey, MD Member, WAO Board of Directors (2010-2011) WAO President (2010-2011) Medical Research Council Clinical Professor of Division of Allergy & Immunology Immunopharmacology Joy McCann Culverhouse Chair in Allergy & Immunology Infection, Inflammation and Immunity University of South Florida College of Medicine School of Medicine James Haley Veterans Administration Medical Center (111D) University of Southampton 13000 Bruce B. Downs Boulevard Level F, South Block Tampa, Florida 33612 Southampton General Hospital USA Tremona Road Southampton SO16 6YD United Kingdom Acknowledgement On behalf of the World Allergy Organization (WAO), the editors and authors of the WAO White Book on Allergy express their gratitude to the charity, Asthma, Allergy, Inflammation Research (AAIR) and Asian Allergy Asthma Foundation (AAAF) for their support in the production of this publication. The Editors of the White book extend their gratitude to His Excellency Dr. APJ Abdul Kalam, Former President of India and Madame Ilora Finlay Baronness of the House of Lords for their Forewords to the White Book and to the International Primary Care Respiratory Group (IPCRG) and European Federation of Allergy and Airways Diseases Patients ‘Associations (EFA) for their supporting statements. -

Bee Venom Allergy in Beekeepers and Their Family Members Ulrich R
Bee venom allergy in beekeepers and their family members Ulrich R. Mu¨ller Purpose of review Introduction To analyze prevalence of allergic sting reactions, including Hymenoptera venom allergy is one of the major reasons the clinical and diagnostic features as well as management for anaphylaxis. Between 1961 and 2000, it caused 120 options in a population heavily exposed to honeybee stings fatalities in Switzerland – an average of three every year such as beekeepers and their family members. [1]. Extrapolated to Western Europe, this corresponds to Recent findings more than 150 fatal Hymenoptera sting reactions every The higher sting frequency is associated with an increased year in this region. Stings by honeybees and vespids are prevalence of allergic sting reactions. Major risk factors for most often responsible for such reactions. Beekeepers allergic sting reactions in beekepers are: fewer than 10 and their family members are heavily exposed to honey- annual stings, an atopic constitution and symptoms of bee stings and are thus at an especially high risk of upper respiratory allergy during work in the beehive. Bee becoming allergic, and therefore are an interesting popu- venom allergic beekeepers have higher levels of bee lation for the study of epidemiology and immunopatho- venom-specific IgG but lower skin sensitivity and bee genesis of venom allergy and the mechanism of its most venom-specific IgE than normally exposed bee venom effective treatment – venom immunotherapy. Finally, allergic patients. Safety of bee venom immunotherapy is owing to the high degree of exposure of this population, higher in beekeepers than in allergic controls, while efficacy indication and protocols for venom immunotherapy may of this treatment is similar in both groups. -

Hymenoptera Sting Challenge of 348 Patients Relation to Subsequent Field
Hymenoptera sting challenge of 348 patients: Relation to subsequent field stings Henk K. van Halteren, MD," Peter-Willem G. van der Linden, MD, PhD, b Sjaak A. Burgers, MD, PhD, c and Anton K. M. Bartelink, MD, PhD a Amersfoort, Haarlem, and Utrecht, The Netherlands' Background: Patients with a history of a serious anaphylactic reaction after a Hymenoptera sting are usually given venom immunotherapy. Because the natural history of Hymenoptera sting anaphylaxis is often of a declining severity, there is a chance of overtreatment. Objective: Identification of patients at risk for a future anaphylactic reaction may reduce the number of patients who need venom immunotherapy. Methods: We investigated the relation between the grade of hypersensitivity to an in-hospital sting challenge and the reaction to a subsequent accidental field sting. From 1982 through 1992, 348 patients" with mild or no symptoms after a sting challenge were not given venom immunotherapy. All patients were asked by letter whether they had experienced a subsequent field sting. In case of" a sting, the severity of the reaction was further evaluated. Results: Information could be obtained from 327patients: 129 had been re-stung, and 110 of them had only had a local reaction. Thirteen patients had experienced mild systemic symptoms, and six patients had experienced serious manifestations. In two of the latter group hypotension was observed. Conclusion: In 95% of patients with a previous anaphylactic reaction, the result of the in- hospital sting challenge provided a good prediction of tolerance to a subsequent Hymenoptera field sting. (J Allergy Clin Immunol 1996;97:1058-63.) Key words: Sting challenge, Hymenoptera, immunotherapy, anaphylccris Most stings by Hymenoptera species only lead to a local reaction: redness, swelling, itching, and Abbreviation used pain. -

Insect Sting Allergy
Insect Sting Allergy Dean Tey Paedi atric Alle rg i st & Immu n ol ogi st Monday 17 June 2010 Insect Sting Allergy 1. Eppgyidemiology 2. Aetiology (meet the insects) 3. Clinical presentation 4. Risk of future systemic reactions 5. Investigations 6. Management a) Prevention b) Local reactions c) Systemic reactions d) Venom immunotherapy Insect Sting Allergy 1. Eppgyidemiology 2. Aetiology (meet the insects) 3. Clinical presentation 4. Risk of future systemic reactions 5. Investigations 6. Management a) Prevention b) Local reactions c) Systemic reactions d) Venom immunotherapy Epidemiology • Large localised reaction – Frequency estimated to be 10% in adults1 • Systemic allergic reactions – Reported by up to 3% of adults2 – S%fSevere sting reactions in up to 1% of children3 1. Golden DBK. Immunol Allergy Clin N Am 2007;17:261-272 2. Golden et al. JAMA 1989;262:240-4. 3. Settipane et al. J Allergy 1972;50:146-50. Epidemiology • Admissions – In Australia, approximately 1200 admissions per year attributed towards hornet, wasp or bee stings (2002-2005) • Fatalities – In Australia, approximately 2 cases per year (20 cases between 1997 -2005). – In USA, >50 cases per year. 1. Bradley C. Australian Institute of Health and Welfare; 2008. Catalog no. INJCAT 110. 2. Liew et al; JACI 2009;123:434-42. 3. Barnard JH. JACI 1973;52:259-64. Liew et al. Anaphylaxis fatalities and admissions in Australia. JACI 2009;123:434-42. Liew et al. Anaphylaxis fatalities and admissions in Australia. JACI 2009;123:434-42. Liew et al. Anaphylaxis fatalities and admissions in Australia. JACI 2009;123:434-42. -

Insect Stings Is Systemic and Severe
Insect Sting Allergy – The Facts Anyone who is stung by a bee or wasp is likely to suffer a painful swelling at the site of the sting. For most people, the sting is not dangerous. Some people also experience an allergic reaction to the venom. For most of these people, the allergic reaction is mild resulting in swelling at the site of the sting. But for a small minority, an allergic reaction to an insect sting can be systemic – that is, affecting parts of the body away from the site of the sting. Systemic allergic reactions are sometimes severe and potentially life-threatening – a condition known as anaphylaxis. If you are in this category, you may find the prospect of being stung very frightening but be assured that there are steps you can take to reduce the risk to yourself. This involves getting medical advice, carrying prescribed medication at all times, and taking precautions to avoid being stung. This Factsheet aims to answer some of the questions you may have if your allergy to insect stings is systemic and severe. Our intention is to help you to minimise risks. The information in this Factsheet includes brief medical references, which are given in brackets. Full references to these documents are listed at the end of the Factsheet. Who is at risk? Anyone can become allergic to an insect sting. People who have other allergies, such as hayfever or food allergies, are not at increased risk of having a severe allergic reaction to an insect sting (Krishna et al. 2011). You are more at risk of severe allergic reactions to insect stings if you have frequent or multiple stings. -
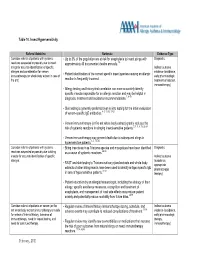
Table 10 Insect Hypersensitivity
Table 10. Insect Hypersensitivity Referral Guideline Rationale Evidence Type Consider referral of patients with systemic • Up to 3% of the population are at risk for anaphylaxis to insect stings with Diagnostic reactions suspected or possibly due to insect approximately 40 documented deaths annually. 1-8 stings for accurate identification of specific Indirect outcome allergen and consideration for venom • evidence (avoidance, immunotherapy (or whole body extract in case of Patient identification of the correct specific insect species causing an allergic early pharmacologic fire ant). reaction is frequently incorrect. treatment of reaction, immunotherapy) • Allergy testing and history-test correlation can more accurately identify specific insects responsible for an allergic reaction and may be helpful in diagnosis, treatment and avoidance recommendations.7, 9-18 • Skin testing is generally preferred over in vitro testing for the initial evaluation of venom-specific IgE antibodies. 4, 5, 13, 15,17-21 • Venom immunotherapy (or fire ant whole body extract) greatly reduces the risk of systemic reactions in stinging insect-sensitive patients. 2, 3, 5, 8, 15, 22-24 • Venom immunotherapy may prevent death due to subsequent stings in hypersensitive patients. 3, 5, 15, 25 Consider referral of patients with systemic • Biting insects such as Triatoma species and mosquitoes have been identified Diagnostic reactions suspected or possibly due to biting as a cause of systemic reactions. 26-30 insects for accurate identification of specific Indirect outcome allergen. • (avoidance, RAST and skin testing to Triatoma salivary gland extracts and whole body appropriate extracts of other biting insects have been used to identify antigen specific IgE 31-41 pharmacologic in sera of hypersensitive patients. -

Medical and Veterinary Entomology
Dhriti Banerjee et al.; Sch J Agric Vet Sci 2015; 2(3B):220-239. Scholars Journal of Agriculture and Veterinary Sciences e-ISSN 2348–1854 Sch J Agric Vet Sci 2015; 2(3B):220-239 p-ISSN 2348–8883 ©Scholars Academic and Scientific Publishers (SAS Publishers) (An International Publisher for Academic and Scientific Resources) Medical and Veterinary Entomology: The good and bad flies that affect human and animal life Dhriti Banerjee1*, Shyamasree Ghosh2, Waliza Ansar3 1Diptera Section, Molecular Systematic Division, Digitization Division & Zoological Survey of India, Ministry of Environment & Forests (Government of India), M Block, New Alipore, Kolkata-700 053 2School of Biological Sciences, National Institute of Science, Education and Research, (NISER), Institute of Physics Campus, Sachivalaya Marg, PO: Sainik School, Bhubaneswar - 751 005, India. 3Behela College, Rabindra Palli, Parnasree, Banamali Naskar Road Kolkata 700060. *Corresponding Authors Name: Dr. Dhriti Banerjee Email: [email protected] Abstract: Medical and veterinary entomology encompasses the study of the insects which are vectors, transmit disease, cause wounds, inject venom and create nuisances together with their application as useful providers of drugs, and model systems for genetic studies. Thus the study on insects and how they affect the human and animal lives have gained importance in recent times. Insect Genomics involving the studies of the whole genome sequences are being developed and gene expression and regulation studies are elucidating their roles in regulating the vector competence. This finds importance in deducing strategies for vector control thereby preventing disease spread that is fatal to man and animal life. In this review we have studied the medical entomology (i) from historical perspective (ii) diverse role of arthropods in causing diseases (iii) common pathogens transmitted by arthropod vectors (iv) major arthropod borne diseases (v) pharmaceutically important insects. -

Insect Venom Hypersensitivity: Experience in a Clinical Immunology/Allergy Service in Singapore B Y H Thong, K P Leong, H H Chng
Original Article Singapore Med J 2005; 46(10) : 535 Insect venom hypersensitivity: experience in a clinical immunology/allergy service in Singapore B Y H Thong, K P Leong, H H Chng ABSTRACT servicemen who developed allergic reactions during the course of duty. The inability to Introduction: To study the profile of patients with identify the causative insect in 50 percent with allergy to the venom of insect stings. sting anaphylaxis limits the role of specific Methods: 31 consecutive cases referred to our immunotherapy in our patients. clinical immunology/allergy outpatient service Keywords: anaphylaxis, insect venom, from June 1, 1998 to June 30, 2002 were reviewed. radioallergosorbent test, skin tests, specific Results: These patients comprised 3.5 percent of immunotherapy 889 cases referred during the study period. Their Singapore Med J 2005; 46(10):535-539 mean age was 28.8 ± 10.5 (range 19-57) years and the majority were males (90.3 percent). INTRODUCTION Of these, 20 (64.5 percent) were Chinese, Insect venom hypersensitivity is often an four (12.9 percent) were Malays and seven immunoglobulin (Ig) E-mediated allergic reaction (22.6 percent) were of other races. 19 patients to the venom of stinging insects belonging to the (61.3 percent) were men from the uniformed order Hymenoptera, which includes bees, wasps and services including 12 (63.2 percent) full-time ants. The diagnosis rests on two criteria: a definitive National Servicemen. 71 percent (22 patients) clinical history that temporally associates an allergic were stung for the first time. Urticaria (22 cases, reaction with an insect sting, and the detection of 71.0 percent), dyspnoea (13, 41.9 percent), venom specific IgE on mast cells in the skin and/ angioedema (12, 38.7 percent) and syncope or blood of the individual by using a confirmatory (ten, 32.3 percent) were the most common skin test or serological assay(1). -

Insect Sting Allergy
Allergy and Immunology Insect Sting Allergy What is insect sting allergy? In Australia the most common insect sting allergy in children is to bee stings. Reactions can also occur to wasps and jack jumper ants. Insect allergy can cause a mild or severe allergic reaction. The allergic reaction is due to IgE allergy antibodies. What sorts of allergic reactions are there? Most children when stung by an insect develop a small area of redness and pain at the insect sting site. This is a normal reaction and does not mean your child is allergic. More marked reactions are of two types; 1 Generalised reactions 2 Large local reactions 1 Generalised reactions Children have a different pattern of insect sting allergy than adults. Bee sting allergy is more common in children. However, severe reactions are less common than in adults. Bee sting reactions which are mild, generalised reactions affecting only the skin occur in about 60% of bee sting allergic children. Children with mild generalised reactions rarely get worse and do not need a course of injections with the aim of reducing the reactions (bee venom immunotherapy). Generalised reactions can become progressively more severe with each sting but this is the exception rather than the rule and occurs in less than 1% of patients. Children with more severe generalized reactions which affect the skin and other systems (for example the breathing passages or less commonly the blood pressure) have a 50- 70% chance of a similar reaction if stung again even many years later. For this reason these children are often treated with a series of injections of bee venom (bee venom immunotherapy). -

Insect Sting Allergy – the Facts
Insect sting allergy – the facts Anyone who is stung by a bee or wasp is likely to suffer a painful swelling at the site of the sting. For most people, the sting is not dangerous. Some people also experience an allergic reaction to the venom. For most of these people, the reaction is mild resulting in swelling at the site of the sting. But for a small minority, an allergic reaction to an insect sting can be systemic – that is, affecting parts of the body away from the site of the sting. Systemic reactions are sometimes severe and potentially life-threatening – this is known as anaphylaxis. If you are in that category, you may find the prospect of being stung very frightening, but be assured that there are steps you can take to reduce the risk to yourself. This involves getting medical advice, carrying prescribed medication at all times, and taking precautions to avoid being stung. Anyone can become allergic to an insect sting – not only people who already have allergies such as hayfever or food allergies. The first sting is not the one that causes the problem, but it may cause sensitisation – the stage at which the person becomes allergic. If sensitisation occurs, it is a later sting that will provoke the symptoms. Being stung by one type of insect will not make you allergic to the venom of another. This fact sheet aims to answer some of the questions you may have if your allergy to insect stings is systemic and severe. Our intention is to help you to minimise risks. -

Mosquito Bites and Bee Stings
Mosquito Bites and Bee Stings Symptoms, Pathology, and Remedies – as seen through the wisdom of Western Medicine, Homeopathy, and Ayurveda Katrina A Johnson May 2012 — Table of Contents — Introduction i Morphology and Physiology of Mosquitoes and Bees 1 Mosquito Bites 1 Bee Stings 5 Mosquito Bite Reactions vs. Bee Sting Reactions 7 Anaphylaxis 7 Western Medical Treatment of Mosquito Bites and Bee Stings 8 Western Medical Treatments for Mosquito Bites and Bee Stings 8 Western Herbal Treatment of Mosquito Bites and Bee Stings 10 Western Herbal Remedies for Mosquito Bites and Bee Stings 10 Homeopathic Understanding of Mosquito Bites and Bee Stings 12 Homeopathic Remedies for Mosquito Bites and Bee Stings 13 Ayurvedic Understanding of Mosquito Bites and Bee Stings 14 Ancient Wisdom from the Ashtanga Hrdayam 16 Ancient Wisdom from the Sushruta Samhita 18 Ancient Wisdom from the Charaka Samhita 20 Charaka’s Remedies for Mosquito Bites and Bee Stings 23 Additional Ayurvedic Remedies for Mosquito Bites and Bee Stings 26 Conclusions 29 Endnotes / References / Journal Abstracts 35 — Introduction — This research paper was born of two impulses: the first, a general interest in assembling a personal first aid kit for travel within the United States; the second, a history of multiple bee stings and the question of how best to treat them. My desire for the first aid kit was fueled by personal reasons. When I travel I carry a rudimentary assortment of things such as band-aids, ibuprofen, skin salve, peppermint tea, and saline wash. This is a decent start, but I wanted to round it out to include remedies for the commonplace travel complaints that I occasionally experience (nausea, diarrhea, bug bites/bee stings, insomnia, and constipation), and I wanted to be more knowledge-base grounded in the choices of what I included in my travel first aid kit. -

TREATMENT of SEVERE ALLERGIC REACTION a Protocol for Training
DHS: PUBLIC HEALTH DIVISION TREATMENT OF SEVERE ALLERGIC REACTION A Protocol for Training Revised January 2008 Authorized for use by the Oregon Department of Human Services, Public Health Division Independent. Healthy. Safe. If you need more information on Epinephrine and/or its use, please contact: Mellony Bernal 971-673-1221 [email protected]. For additional copies or if you need this document in an alternate format, contact: Dan Nielsen 971-673-1230 FAX: (503) 872-6738 [email protected] TABLE of CONTENTS I. INTRODUCTION ........................................................... 1 II. BACKGROUND ............................................................. 2 A. An explanation of the law and rules .......................... 2 B. The training program ................................................ 2 C. Who can be trained .................................................. 3 III. WHAT IS ANAPHYLAXIS? ............................................ 4 A. Overview of the causes of anaphylaxis ..................... 4 B. Insect stings .............................................................. 5 C. Foods ........................................................................ 7 D. Medications.............................................................. 9 E. Other allergens ........................................................ 10 V. IDENTIFYING THE SENSITIVE INDIVIDUAL ............... 11 VI. RECOGNIZING ANAPHYLAXIS ................................. 12 VII. TREATMENT FOR ANAPHYLAXIS ............................. 14