Ketamine: Translating Mechanistic Discoveries Into the Next Generation of Glutamate Modulators for Mood Disorders
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Misuse of Drugs Act (MEI 2013).Fm
LAWS OF BRUNEI CHAPTER 27 MISUSE OF DRUGS 7 of 1978 9 of 1979 1984 Edition, Chapter 27 Amended by 10 of 1982 S 27/1982 S 20/1984 S 8/1987 S 36/1987 S 20/1989 S 24/1991 S 20/1992 S 28/1994 S 42/1998 S 60/1999 2001 Edition, Chapter 27 Amended by S 7/2002 S 59/2007 S 5/2008 S 12/2010 S 12/2012 REVISED EDITION 2013 B.L.R.O. 2/2013 LAWS OF BRUNEI Misuse of Drugs CAP. 27 1 LAWS OF BRUNEI REVISED EDITION 2013 CHAPTER 27 MISUSE OF DRUGS ARRANGEMENT OF SECTIONS Section PART I PRELIMINARY 1. Citation. 2. Interpretation. 2A. Appointment of Director and other officers of Bureau. 2B. Public servants. 2C. Powers of investigations of Bureau. 2D. Use of weapons. PART II OFFENCES INVOLVING CONTROLLED DRUGS 3. Trafficking in controlled drug. 3A. Possession for purpose of trafficking. 4. Manufacture of controlled drug. 5. Importation and exportation of controlled drug. B.L.R.O. 2/2013 LAWS OF BRUNEI 2 CAP. 27 Misuse of Drugs 6. Possession and consumption of controlled drug. 6A. Consumption of controlled drug outside Brunei Darussalam by permanent resident. 6B. Place of consumption need not be stated or proven. 7. Possession of pipes, utensils etc. 8. Cultivation of cannabis, opium and coca plants. 8A. Manufacture, supply, possession, import or export of equipment, materials or substances useful for manufacture of controlled drugs. 8B. Regulations and controlled substances. 9. Responsibilities of owners and tenants etc. 10. Abetments and attempts punishable as offences. 11. -

A Guide to Glutamate Receptors
A guide to glutamate receptors 1 Contents Glutamate receptors . 4 Ionotropic glutamate receptors . 4 - Structure ........................................................................................................... 4 - Function ............................................................................................................ 5 - AMPA receptors ................................................................................................. 6 - NMDA receptors ................................................................................................. 6 - Kainate receptors ............................................................................................... 6 Metabotropic glutamate receptors . 8 - Structure ........................................................................................................... 8 - Function ............................................................................................................ 9 - Group I: mGlu1 and mGlu5. .9 - Group II: mGlu2 and mGlu3 ................................................................................. 10 - Group III: mGlu4, mGlu6, mGlu7 and mGlu8 ............................................................ 10 Protocols and webinars . 11 - Protocols ......................................................................................................... 11 - Webinars ......................................................................................................... 12 References and further reading . 13 Excitatory synapse pathway -

RAP-MD-05 GLYX13-C-203 Protocol Amendment 3 Final-R
NCT02192099 Study ID: GLYX13‐C‐203 Title: Open Label Extension for Subjects with Inadequate/Partial Response to Antidepressants during the Current Episode of Major Depressive Disorder Previously Treated with Rapastinel (GLYX‐13) (Extension of GLYX13‐C‐202, NCT01684163) Protocol Amendment 3 Date: 23 Nov 2016 Clinical Study Protocol Protocol Number: GLYX13-C-203 Study Drug: Rapastinel (GLYX-13) Injection (rapastinel Injection) FDA ID: 107,974 Clinicaltrials.gov: Title: Open Label Extension for Subjects with Inadequate/Partial Response to Antidepressants during the Current Episode of Major Depressive Disorder Previously Treated with Rapastinel (GLYX-13) (Extension of GLYX13-C-202, NCT01684163) Study Phase: 2 Sponsor: Naurex, Inc, an affiliate of Allergan, plc. Date: Amendment 3 dated 23 November 2016 Replaces Amendment 2 dated 20 January 2015 This document is a confidential communication from Naurex, Inc, an affiliate of Allergan, plc.. Acceptance of this document constitutes an agreement by the recipient(s) that no information contained herein will be published or disclosed without prior written approval from Allergan, plc., except that this document may be disclosed to appropriate Institutional Review Boards (IRBs) under the condition that they are also required to maintain confidentiality. Naurex, Inc, an affiliate of Allergan, plc. Protocol GLYX13-C-203 INVESTIGATOR SIGNATURE PAGE The signature of the investigator below constitutes his/her approval of this protocol and provides the necessary assurances that this study will be conducted according to all stipulations of the protocol as specified in both the clinical and administrative sections, including all statements regarding confidentiality. This trial will be conducted in compliance with the protocol and all applicable regulatory requirements, in accordance with Good Clinical Practices (GCPs), including International Conference on Harmonization (ICH) Guidelines, and in general conformity with the most recent version of the Declaration of Helsinki. -

Interplay Between Gating and Block of Ligand-Gated Ion Channels
brain sciences Review Interplay between Gating and Block of Ligand-Gated Ion Channels Matthew B. Phillips 1,2, Aparna Nigam 1 and Jon W. Johnson 1,2,* 1 Department of Neuroscience, University of Pittsburgh, Pittsburgh, PA 15260, USA; [email protected] (M.B.P.); [email protected] (A.N.) 2 Center for Neuroscience, University of Pittsburgh, Pittsburgh, PA 15260, USA * Correspondence: [email protected]; Tel.: +1-(412)-624-4295 Received: 27 October 2020; Accepted: 26 November 2020; Published: 1 December 2020 Abstract: Drugs that inhibit ion channel function by binding in the channel and preventing current flow, known as channel blockers, can be used as powerful tools for analysis of channel properties. Channel blockers are used to probe both the sophisticated structure and basic biophysical properties of ion channels. Gating, the mechanism that controls the opening and closing of ion channels, can be profoundly influenced by channel blocking drugs. Channel block and gating are reciprocally connected; gating controls access of channel blockers to their binding sites, and channel-blocking drugs can have profound and diverse effects on the rates of gating transitions and on the stability of channel open and closed states. This review synthesizes knowledge of the inherent intertwining of block and gating of excitatory ligand-gated ion channels, with a focus on the utility of channel blockers as analytic probes of ionotropic glutamate receptor channel function. Keywords: ligand-gated ion channel; channel block; channel gating; nicotinic acetylcholine receptor; ionotropic glutamate receptor; AMPA receptor; kainate receptor; NMDA receptor 1. Introduction Neuronal information processing depends on the distribution and properties of the ion channels found in neuronal membranes. -

(NMDA) Receptor Encephalitis Is an Autoimmune Seizures and Autonomic Dysfunction
Nonprofit Organization U.S. Postage PAID Twin Cities, MN VOLUME 23, NUMBER 4 200 University Ave. E. Permit No. 5388 St. Paul, MN 55101 651-291-2848 ADDRESSwww.gillettechildrens.org SERVICE REQUESTED VOLUME 23, NUMBER 4 2014 A Pediatric Perspective focuses on specialized topics in pediatrics, orthopedics, neurology, neurosurgery and rehabilitation medicine. Diagnosing and Treating Anti- KEY INSIGHTS To subscribe to or unsubscribe from A Pediatric Perspective, please send an email to [email protected]. N-Methyl-D-Aspartate (NMDA) ■ Anti-NMDA receptor encephalitis Editor-in-Chief – Steven Koop, M.D. is a relatively common and treatable Editor – Ellen Shriner Receptor Encephalitis cause of encephalitis in pediatric Designers – Becky Wright, Kim Goodness patients. Photographers – Anna Bittner, Amanda Moen, M.D., pediatric neurologist Paul DeMarchi ■ Common symptoms include person- Copyright 2014. Gillette Children’s Specialty ality change, abnormal movements, Amanda Moen, M.D. Healthcare. All rights reserved. Anti-N-methyl-D-aspartate (NMDA) receptor encephalitis is an autoimmune seizures and autonomic dysfunction. condition in which the body produces antibodies that act against receptors in ■ If these symptoms are present, an the brain, resulting in both neurologic and psychiatric symptoms. Common urgent child neurology evaluation is symptoms include personality change, psychosis, abnormal movements, indicated and an evaluation for anti- To make a referral, call 651-325-2200 or seizures and autonomic dysfunction. The antibodies associated with this NMDA receptor encephalitis should Pediatric neurologist Amanda Moen, M.D., treats children 855-325-2200 (toll-free). condition were first identified in 2005, and since then it has become recognized be initiated. who have epilepsy and other neurological conditions. -

From NMDA Receptor Hypofunction to the Dopamine Hypothesis of Schizophrenia J
REVIEW The Neuropsychopharmacology of Phencyclidine: From NMDA Receptor Hypofunction to the Dopamine Hypothesis of Schizophrenia J. David Jentsch, Ph.D., and Robert H. Roth, Ph.D. Administration of noncompetitive NMDA/glutamate effects of these drugs are discussed, especially with regard to receptor antagonists, such as phencyclidine (PCP) and differing profiles following single-dose and long-term ketamine, to humans induces a broad range of exposure. The neurochemical effects of NMDA receptor schizophrenic-like symptomatology, findings that have antagonist administration are argued to support a contributed to a hypoglutamatergic hypothesis of neurobiological hypothesis of schizophrenia, which includes schizophrenia. Moreover, a history of experimental pathophysiology within several neurotransmitter systems, investigations of the effects of these drugs in animals manifested in behavioral pathology. Future directions for suggests that NMDA receptor antagonists may model some the application of NMDA receptor antagonist models of behavioral symptoms of schizophrenia in nonhuman schizophrenia to preclinical and pathophysiological research subjects. In this review, the usefulness of PCP are offered. [Neuropsychopharmacology 20:201–225, administration as a potential animal model of schizophrenia 1999] © 1999 American College of is considered. To support the contention that NMDA Neuropsychopharmacology. Published by Elsevier receptor antagonist administration represents a viable Science Inc. model of schizophrenia, the behavioral and neurobiological KEY WORDS: Ketamine; Phencyclidine; Psychotomimetic; widely from the administration of purportedly psychot- Memory; Catecholamine; Schizophrenia; Prefrontal cortex; omimetic drugs (Snyder 1988; Javitt and Zukin 1991; Cognition; Dopamine; Glutamate Jentsch et al. 1998a), to perinatal insults (Lipska et al. Biological psychiatric research has seen the develop- 1993; El-Khodor and Boksa 1997; Moore and Grace ment of many putative animal models of schizophrenia. -

Presence of Methoxetamine in Drug Samples Delivered As Ketamine for Substance Analysis
Is this really ketamine? Presence of methoxetamine in drug samples delivered as ketamine for substance analysis P. Quintana2, Á. Palma1,5, M. Grifell1,2, J. Tirado3,5, C. Gil2, M. Ventura2, I. Fornís3, S. López2, V. Perez1,5, M. Torrens1,3,5, M. Farré3,4,5, L. Galindo1,3,5 1Institut de Neuropsiquiatria i Addiccions, Parc de Salut Mar, Barcelona, Spain 2Asocicación Bienestar y Desarrollo, Energy Control, Barcelona, Spain 3IMIM (Hospital del Mar Medical Research Institute), Barcelona, Spain 4Hospital Germans Trías i Pujol, Servei de Farmacología Clínica, Barcelona, Spain 5Departament de Psiquiatria, Universitat Autònoma de Barcelona, Barcelona, Spain Introduction New psychoactive substances (NPS) are drugs that have recently become available, and are not world-wide regulated. They often intend to mimic the effect of controlled drugs (1), becoming a public health concern. In 2014, 101 new substances were reported for the first time in the EU (2). Methoxetamine, an NPS, is a dissociative hallucinogenic acting as a NMDA receptor antagonist, clinically and pharmacologically similar to Ketamine (3). It differs from ketamine on its higher potency and duration as well as on its possible serotonergic activity, probably leading to more severe side effects. It has been one of the 6 substances requiring risk assessment by EMCDDA during 2014(3), and several case reports have described fatal outcomes related with its use(4). Objective Material and methods The aim of this study was 1) to explore the presence of methoxetamine from the All samples analyzed from January 2010 to March 2015 in which methoxetamine was samples handled to, and analyzed by Energy Control and 2) to determine whether found were studied. -

Poster Session III, December 6, 2017
Neuropsychopharmacology (2017) 42, S476–S652 © 2017 American College of Neuropsychopharmacology. All rights reserved 0893-133X/17 www.neuropsychopharmacology.org Poster Session III a high-resolution research tomograph (HRRT), and struc- Palm Springs, California, December 3–7, 2017 tural (T1) MRI using a 3-Tesla scanner. PET data analyses were carried out using the validated 2-tissue compartment Sponsorship Statement: Publication of this supplement is model to determine the total volume of distribution (VT) of sponsored by the ACNP. [18F]FEPPA. Individual contributor disclosures may be found within the Results: Results show significant inverse associations (con- abstracts. Part 1: All Financial Involvement with a pharma- trolling for rs6971 genotype) between neuroinflammation ceutical or biotechnology company, a company providing (VTs) and cortical thickness in the right medial prefrontal clinical assessment, scientific, or medical products or compa- cortex [r = -.562, p = .029] and the left dorsolateral nies doing business with or proposing to do business with prefrontal cortex [r = -.629, p = .012](p values are not ACNP over past 2 years (Calendar Years 2014–Present); Part 2: corrected for multiple comparisons). No significant associa- Income Sources & Equity of $10,000 per year or greater tions were found with surface area. (Calendar Years 2014 - Present): List those financial relation- Conclusions: These results, while preliminary, suggest links ships which are listed in part one and have a value greater than between the microglial activation/neuroinflammation and $10,000 per year, OR financial holdings that are listed in part cortical thickness in the dorsolateral prefrontal cortex and one and have a value of $10,000 or greater as of the date of medial prefrontal cortex in AD patients. -

Pharmacogenetics of Ketamine Metabolism And
Pharmacogenetics of Ketamine Metabolism and Immunopharmacology of Ketamine Yibai Li B.HSc. (Hons) Discipline of Pharmacology, School of Medical Sciences, Faculty of Health Sciences, The University of Adelaide September 2014 A thesis submitted for the Degree of PhD (Medicine) Table of contents TABLE OF CONTENTS .............................................................................................. I LIST OF FIGURES ....................................................................................................IV LIST OF TABLES ......................................................................................................IV ABSTRACT ............................................................................................................... V DECLARATION .......................................................................................................VIII ACKNOWLEDGEMENTS ..........................................................................................IX ABBREVIATIONS .....................................................................................................XI CHAPTER 1. INTRODUCTION .................................................................................. 1 1.1 A historical overview of ketamine ........................................................................................ 1 1.2 Structure and Chemistry ....................................................................................................... 3 1.3 Classical analgesic mechanisms of ketamine ................................................................... -

Neurochemical Mechanisms Underlying Alcohol Withdrawal
Neurochemical Mechanisms Underlying Alcohol Withdrawal John Littleton, MD, Ph.D. More than 50 years ago, C.K. Himmelsbach first suggested that physiological mechanisms responsible for maintaining a stable state of equilibrium (i.e., homeostasis) in the patient’s body and brain are responsible for drug tolerance and the drug withdrawal syndrome. In the latter case, he suggested that the absence of the drug leaves these same homeostatic mechanisms exposed, leading to the withdrawal syndrome. This theory provides the framework for a majority of neurochemical investigations of the adaptations that occur in alcohol dependence and how these adaptations may precipitate withdrawal. This article examines the Himmelsbach theory and its application to alcohol withdrawal; reviews the animal models being used to study withdrawal; and looks at the postulated neuroadaptations in three systems—the gamma-aminobutyric acid (GABA) neurotransmitter system, the glutamate neurotransmitter system, and the calcium channel system that regulates various processes inside neurons. The role of these neuroadaptations in withdrawal and the clinical implications of this research also are considered. KEY WORDS: AOD withdrawal syndrome; neurochemistry; biochemical mechanism; AOD tolerance; brain; homeostasis; biological AOD dependence; biological AOD use; disorder theory; biological adaptation; animal model; GABA receptors; glutamate receptors; calcium channel; proteins; detoxification; brain damage; disease severity; AODD (alcohol and other drug dependence) relapse; literature review uring the past 25 years research- science models used to study with- of the reasons why advances in basic ers have made rapid progress drawal neurochemistry as well as a research have not yet been translated Din understanding the chemi- reluctance on the part of clinicians to into therapeutic gains and suggests cal activities that occur in the nervous consider new treatments. -

Functional Kainate-Selective Glutamate Receptors in Cultured Hippocampal Neurons (Excitatory Amino Acid Receptors/Hippocampus) JUAN LERMA*, ANA V
Proc. Natl. Acad. Sci. USA Vol. 90, pp. 11688-11692, December 1993 Neurobiology Functional kainate-selective glutamate receptors in cultured hippocampal neurons (excitatory amino acid receptors/hippocampus) JUAN LERMA*, ANA V. PATERNAIN, JosE R. NARANJO, AND BRITT MELLSTR6M Departamento de Plasticidad Neural, Instituto Cajal, Consejo Superior de Investigaciones Cientfficas, Avenida Doctor Arce 37, 28002-Madrid, Spain Communicated by Michael V. L. Bennett, September 15, 1993 ABSTRACT Glutamate mediates fast synaptic transmis- experiments, the regional distribution of high-affinity sion at the majority of excitatory synapses throughout the [3H]kainate binding sites does not match the AMPA receptor central nervous system by interacting with different types of distribution but corresponds well to the brain areas with high receptor channels. Cloning of glutamate receptors has pro- susceptibility to the neurotoxic actions of kainate (e.g., vided evidence for the existence of several structurally related hippocampal CA3 field) (13). However, patch-clamp record- subunit families, each composed of several members. It has ings from adult hippocampal neurons have revealed that been proposed that KA1 and KA2 and GluR-5, GluR-6, and native glutamate receptors are similar to the AMPA-type GluR-7 families represent subunit classes of high-affinity kain- recombinant glutamate receptors expressed from cDNA ate receptors and that in vivo different kainate receptor sub- clones but have failed so far to detect receptor channels ofthe types might be constructed from these subunits in heteromeric kainate type (14, 15). The only apparently high-affinity kain- assembly. However, despite some indications from autoradio- ate receptor channels have been found in the peripheral graphic studies and binding data in brain membranes, no nervous system (16, 17), although they are also activated by functional pure kainate receptors have so far been detected in AMPA. -
Rapastinel Flop Leaves Allergan in a Hole
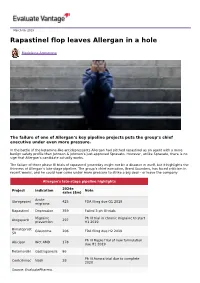
March 06, 2019 Rapastinel flop leaves Allergan in a hole Madeleine Armstrong The failure of one of Allergan’s key pipeline projects puts the group’s chief executive under even more pressure. In the battle of the ketamine-like antidepressants Allergan had pitched rapastinel as an agent with a more benign safety profile than Johnson & Johnson’s just-approved Spravato. However, unlike Spravato, there is no sign that Allergan’s candidate actually works. The failure of three phase III trials of rapastinel yesterday might not be a disaster in itself, but it highlights the thinness of Allergan’s late-stage pipeline. The group’s chief executive, Brent Saunders, has faced criticism in recent weeks, and he could now come under more pressure to strike a big deal – or leave the company. Allergan's late-stage pipeline highlights 2024e Project Indication Note sales ($m) Acute Ubrogepant 425 FDA filing due Q1 2019 migraine Rapastinel Depression 359 Failed 3 ph III trials Migraine Ph III trial in chronic migraine to start Atogepant 297 prevention H1 2019 Bimatoprost Glaucoma 206 FDA filing due H2 2019 SR Ph III Maple trial of new formulation Abicipar Wet AMD 178 due H1 2019 Relamorelin Gastroparesis 86 Ph III Aurora trial due to complete Cenicriviroc Nash 28 2020 Source: EvaluatePharma. Activist shareholders have long been agitating for change at Allergan, and the latest failure will only provide more ammunition to those who want to split the roles of chairman and chief exec amid dissatisfaction with the group's acquisition strategy, and with its stock hovering around a five-year low.