Genetic Variation, Heritability and Genotype Environment
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Newsletter for July 2003, Volume 33
CHINESE CLAYART NEWSLETTER July 2003, Vol. 33 FEATURE Jingdezhen, China - Celebrate the Millennium of the Porcelain Town PROFILE Yixing Ceramic Art Traveling Exhibition, USA 2003-2008 TRAVEL Chinese Ceramic Cultural Travel and Exchange 2004 ACTIVITIES Workshops hosted by the Pottery Workshop Gallery, Hong Kong LETTERS Letter from NCECA, March 24, 2003 STORE New Items and Books of The Chinese Clay Art Web Store, and the National Distributors CHINESE CLAYART " Chinese Clayart" is a newsletter emailed monthly to professional ceramic artists who want to know about ceramic art in China and things related. This newsletter will be a bridge between China and Western countries for the ceramic arts. Comments and suggestions are very welcome. (Copyright 2000, The Chinese Ceramic Art Council, USA. All rights reserved) The Chinese Ceramic Art Council, USA. P.O.Box 64392, Sunnyvale, CA 94088, USA Tel. 408-777-8319, Fax. 408-777-8321, Email: [email protected] www.chineseclayart.com Chief Editor, Guangzhen "Po" Zhou English Editor, Deborah Bouchette TOP FEATURE Jingdezhen, China - Celebrate the Millennium of the Porcelain Town Together with local companies, the government of China is going to invest 60 billion RMB Yuan (equal to about $7.25 billion) to build or restore 20 huge projects. These huge projects include the Ceramic Town of China, the Ceramic Art Center of China, the Imperial Kiln Museum, the Sculpture Garden of World Celebrities, and many traditional residential houses and porcelain-ware studios to attract tourists. All of these programs will be completed in October 2004. JINGDEZHEN Located in northeast Jiangxi Province, Jingdezhen is renowned as the "Capital of Porcelain" and has over 1,700 years' ceramic production history. -

Research on the Relationship Between Green Development and Fdi in Jiangxi Province
2019 International Seminar on Education, Teaching, Business and Management (ISETBM 2019) Research on the Relationship between Green Development and Fdi in Jiangxi Province Zejie Liu1, a, Xiaoxue Lei2, b, Wenhui Lai1, c, Zhenyue Lu1, d, Yuanyuan Yin1, e 1School of Business, Jiangxi Normal University, Nanchang 330022, China 2 School of Financial and Monetary, Jiangxi Normal University, Nanchang 330022, China Keywords: Jiangxi province, City, Green development, Fdi Abstract: Firstly, based on factor analysis method, this paper obtained the green development comprehensive index and its changing trend of 11 prefecture level cities in Jiangxi Province. Secondly, by calculating Moran's I index, it made an empirical analysis of the spatial correlation of FDI between 11 prefecture level cities in Jiangxi Province, both globally and locally. Finally, this paper further tested the relationship between FDI and green development index by establishing multiple linear regression equation. The results show that the overall green development level of Jiangxi Province shows a steady upward trend, but the development gap between 11 prefecture level cities in Jiangxi Province is large, and the important green development indicators have a significant positive impact on FDI. 1. Introduction As one of the important provinces rising in Central China, Jiangxi has made remarkable achievements in the protection of ecological environment and the promotion of economic benefits while vigorously promoting green development and actively introducing FDI. Data shows that since the first batch of FDI was introduced in 1984, Jiangxi Province has attracted 12.57 billion US dollars of FDI in 2018, with an average annual growth rate of 19.42%. By the first three quarters of 2019, Jiangxi's actual use of FDI amounted to 9.79 billion US dollars, an increase of 8.1%, ranking at the top of the country. -

Study on the Development Strategy of Ceramic Cultural
Advances in Computer Science Research (ACSR), volume 76 7th International Conference on Education, Management, Information and Mechanical Engineering (EMIM 2017) Study on the Development Strategy of Ceramic Cultural Creative Industry under the Strategy of "One Belt and One Road" - Taking Jingdezhen as an Example Dawei Ke Jingdezhen Ceramic Institute, Jingdezhen, Jiangxi 333403, China [email protected] Keywords: "One Belt and One Road"; Ceramic culture; Creative industry; Development strategy. Abstract. The strategy of "One Belt and One Road" is the national strategy put forward by the state in order to promote the common development of the neighboring countries of the Silk Road. The ceramic as a typical Chinese traditional culture carrier, in the strategic background of "One Belt and One Road", is bound to play a more significant in cultural exchanges and heritage. Millennium porcelain city - Jingdezhen, as an important representative place of China's ceramic culture, have great responsibility for constructing ceramic culture and creative industries, promoting its development. Based on the present situation and significance of the development of Jingdezhen ceramic culture and creative industry under the strategy of "One Belt and One Road", this paper carried out analysis according to the strategic layout and problems existing in the current development, and finally puts forward the development suggestions and countermeasures, in order to communicate with each other. The level of cultural development is an important manifestation of the country's soft power. In recent years, the cultural and creative industries have gradually revealed its role in promoting the world's social and economic development. Partial developed countries are treating the cultural and creative industries as the new forces of leading industry to promote innovation and development and pay more attention to it. -

The Development of Jingdezhen in the View of Cultural Innovation
ISSN 1923-0176 [Print] Studies in Sociology of Science ISSN 1923-0184 [Online] Vol. 3, No. 4, 2012, pp. 45-49 www.cscanada.net DOI:10.3968/j.sss.1923018420120304.ZR0102 www.cscanada.org The Development of Jingdezhen in the View of Cultural Innovation LI Songjie[a],*; WANG Shujing[b]; LI Xinghua[c] [a] Senior Lecturer, Graduate School of Ceramic Aesthetics, Jingdezhen session of the Communist Party of China held in Beijing Ceramic Institute, China. Mainly engaged in History of culture and in October 2011, the Sixth Plenary Session passed the ceramic culture research. [b] Senior Lecturer, Graduate School of Ceramic Aesthetics, Jingdezhen “CPC Central Committee decision” to deepen cultural Ceramic Institute, China. Mainly engaged in British and American restructuring, and promote socialist cultural development Cultural Studies. and prosperity of a number of major issues of scientific [c] Professor, Graduate School of Ceramic Aesthetics, Jingdezhen profiles and profoundly expounded the road of socialist Ceramic Institute, China. Mainly engaged in Study of local culture. * Corresponding author. cultural development, to deepen cultural restructuring and promoting the development of prosperity of socialist Supported by National Natural Science Fund Program of 2010. (No. culture, and efforts to build a socialist culture power. The 41061020). Chinese nation has a brilliant culture of the thousands Received 6 June 2012; accepted 31 October 2012 of years, which is the pride of the Chinese nation, and also a valuable asset of national development. It can not only maintain the self-esteem and self-confidence of a Abstract country, but also is an important force to push forward Jingdezhen porcelain is famous all over the world. -

Universe Pharmaceuticals INC Form FWP Filed 2021-03-01
SECURITIES AND EXCHANGE COMMISSION FORM FWP Filing under Securities Act Rules 163/433 of free writing prospectuses Filing Date: 2021-03-01 SEC Accession No. 0001213900-21-012481 (HTML Version on secdatabase.com) SUBJECT COMPANY Universe Pharmaceuticals INC Mailing Address Business Address 265 JINGJIU AVENUE 265 JINGJIU AVENUE CIK:1809616| IRS No.: 000000000 | State of Incorp.:F4 | Fiscal Year End: 0930 JINGGANGSHAN ECON. JINGGANGSHAN ECON. Type: FWP | Act: 34 | File No.: 333-248067 | Film No.: 21697215 AND TECH. DEV. ZONE AND TECH. DEV. ZONE SIC: 2834 Pharmaceutical preparations JI'AN, JIANGXI F4 343100 JI'AN, JIANGXI F4 343100 86-0796-8403309 FILED BY Universe Pharmaceuticals INC Mailing Address Business Address 265 JINGJIU AVENUE 265 JINGJIU AVENUE CIK:1809616| IRS No.: 000000000 | State of Incorp.:F4 | Fiscal Year End: 0930 JINGGANGSHAN ECON. JINGGANGSHAN ECON. Type: FWP AND TECH. DEV. ZONE AND TECH. DEV. ZONE SIC: 2834 Pharmaceutical preparations JI'AN, JIANGXI F4 343100 JI'AN, JIANGXI F4 343100 86-0796-8403309 Copyright © 2021 www.secdatabase.com. All Rights Reserved. Please Consider the Environment Before Printing This Document Issuer Free Writing Prospectus Filed Pursuant to Rule 433 of the Securities Act of 1933, as amended Registration Statement No. 333-248067 March 1, 2021 Universe Pharmaceuticals INC Free Writing Prospectus This free writing prospectus relates to the proposed public offering of ordinary shares of Universe Pharmaceuticals INC (the “Company”) that is being registered on a Registration Statement on Form F-1 (No. 333-248067) (the “Registration Statement”) and should be read together with the preliminary prospectus dated February 24, 2021, included in the Registration Statement which can be accessed through the following web link: https://www.sec.gov/Archives/edgar/data/1809616/000121390021011334/ea136119-f1a3_universepharma.htm The Company has filed the Registration Statement with the SEC for the offering to which this communication relates. -

An Artist's Life in Jingdezhen, China
PERSPECTIVE AN ARTIST’S LIFE IN JINGDEZHEN, CHINA Gary Erickson* or the past nine years, I have been traveling to China to spend my summers in Jingdezhen, known as the “porcelain city of FChina.” This historic city has been producing ceramics for seventeen hundred years, with the fi rst porcelains invented over one thousand years ago. Living and creating abroad has both challenged and rewarded me as an artist. The translation from low-temperature earthenware sculpture created in my Minnesota studio to pure white high-temperature porcelain in Jingdezhen has been a fascinating journey and almost as daunting as trying to learn the Chinese language. For years I had been taught about kaolin in materials classes and used it in slips and glazes. When I hiked over a portion of Mount Gaoling for the fi rst time, I visualized workers bringing kaolin out of the cave mines and down the well-worn path to the river for processing. The one thousand years of effort by miners, clay makers, throwers, mold makers, painters, and kiln fi rers fl ooded my mind. In my Jingdezhen studio I use the porcelain for the same properties that artists have appreciated for centuries. I am amused by the fact that someone like me—a six- foot-fi ve-inch-tall, English-speaking Midwesterner—is now connected to the history of Chinese porcelain and part of the harmonious fabric of the porcelain industry. The author standing next to a giant teapot created in one of the “bigware” factories. This piece was not made for a customer but as testament to the scale at which this studio can work. -

Production and Trade of Porcelain in China, 1000-1500
Production and Trade of Porcelain in China, 1000-1500 Shelagh Vainker Ashmolean Museum, Oxford Email: [email protected] Just as the Song dynasty (960-1279) has been identified by economic historians as a peak after which no significant developments took place, so it is a period of culmination in the manufacture of ceramics. Between the 10th and 12th centuries, green, black or white high- fired wares that had been produced for centuries were made with finer bodies, smoother and more complex glazes and in a greater range of shapes than ever before, and in unprecedented quantities. They also became admired and moreover collected as objects of aesthetic, cultural and monetary value, a practice that had previously been restricted to works of art such as calligraphy and painting, or to the jades and bronze vessels associated with high antiquity and the authority to rule. This was also however the period in which was established China’s most enduring and famous kiln site, Jingdezhen. To that extent the period is not only a technological peak, but a pivotal one during which the centre of the ceramics industry began its shift from north to south China. At the time when the northern kilns were producing pieces for the imperial court and wares that would be adopted into the canon of connoisseur’s collectibles, southern kilns were making pots for everyday local use, and for export. In many instances, these imitated the northern wares in both technology and style; all were part of a country-wide industry with a distribution of manufacturing centres that was unprecedented and has not been repeated, for it is notable that during the pre-eminence of the north in potting, the south was also rich in kiln sites and products, while once the shift south had occurred no significant industry continued in north China. -

The Protection of Intangible Cultural Heritage from the Perspective of Art Anthropology: Case Study of Jingdezhen’S Folk Ceramic Craft
Frontiers in Educational Research ISSN 2522-6398 Vol. 2, Issue 4: 79-82, DOI: 10.25236/FER.034021 The Protection of Intangible Cultural Heritage from the Perspective of Art Anthropology: Case Study of Jingdezhen’s Folk Ceramic Craft Yang Liu Faculty of Humanities, Charles University, Prague 02101–02117, Czech Republic ABSTRACT.Ceramic as tangible material culture, it is generally not in the category of intangible culture, however, the inheritance process of Jingdezhen's folk ceramic craft through “oral teaching” between masters and apprentices includes different kinds of intangible cultural elements. From the angle of the protection of intangible cultural heritage and art anthropology, this article tries to discuss the intangible cultural factors contained in Jingdezhen's folk ceramic craft, combined with related current situations of rescuing and protecting Jingdezhen's folk porcelain techniques, and propose the slogan “saving people and saving environment” to maintain Jingdezhen’s folk ceramic craft. How to make ceramic craft be better disseminated and inherited, and what new forms can absorb in the future? It is also a subject that needs our further investigation in the future. In so doing, I hope this article will contribute to a growing body of attention in contemporary intellectual history on intangible culture inheritance as well as the ideas of anthropology. KEYWORDS: Art anthropology; folk ceramic craft; intangible cultural heritage; Jingdezhen; protection and inheritance 1. Introduction On October 17, 2003, UNESCO passed the Convention for the Safeguarding of the Intangible Cultural Heritage, officially named and published the five basic contents of the protection of intangible cultural heritage, traditional handicraft ranked among them[1]. -
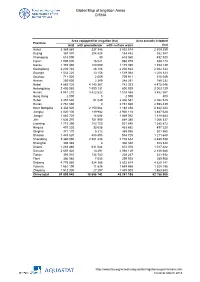
Global Map of Irrigation Areas CHINA
Global Map of Irrigation Areas CHINA Area equipped for irrigation (ha) Area actually irrigated Province total with groundwater with surface water (ha) Anhui 3 369 860 337 346 3 032 514 2 309 259 Beijing 367 870 204 428 163 442 352 387 Chongqing 618 090 30 618 060 432 520 Fujian 1 005 000 16 021 988 979 938 174 Gansu 1 355 480 180 090 1 175 390 1 153 139 Guangdong 2 230 740 28 106 2 202 634 2 042 344 Guangxi 1 532 220 13 156 1 519 064 1 208 323 Guizhou 711 920 2 009 709 911 515 049 Hainan 250 600 2 349 248 251 189 232 Hebei 4 885 720 4 143 367 742 353 4 475 046 Heilongjiang 2 400 060 1 599 131 800 929 2 003 129 Henan 4 941 210 3 422 622 1 518 588 3 862 567 Hong Kong 2 000 0 2 000 800 Hubei 2 457 630 51 049 2 406 581 2 082 525 Hunan 2 761 660 0 2 761 660 2 598 439 Inner Mongolia 3 332 520 2 150 064 1 182 456 2 842 223 Jiangsu 4 020 100 119 982 3 900 118 3 487 628 Jiangxi 1 883 720 14 688 1 869 032 1 818 684 Jilin 1 636 370 751 990 884 380 1 066 337 Liaoning 1 715 390 783 750 931 640 1 385 872 Ningxia 497 220 33 538 463 682 497 220 Qinghai 371 170 5 212 365 958 301 560 Shaanxi 1 443 620 488 895 954 725 1 211 648 Shandong 5 360 090 2 581 448 2 778 642 4 485 538 Shanghai 308 340 0 308 340 308 340 Shanxi 1 283 460 611 084 672 376 1 017 422 Sichuan 2 607 420 13 291 2 594 129 2 140 680 Tianjin 393 010 134 743 258 267 321 932 Tibet 306 980 7 055 299 925 289 908 Xinjiang 4 776 980 924 366 3 852 614 4 629 141 Yunnan 1 561 190 11 635 1 549 555 1 328 186 Zhejiang 1 512 300 27 297 1 485 003 1 463 653 China total 61 899 940 18 658 742 43 241 198 52 -

Jiangxi Shangrao Early Childhood Education Demonstration Program (RRP PRC 51434)
Jiangxi Shangrao Early Childhood Education Demonstration Program (RRP PRC 51434) JIANGXI SHANGRAO EARLY CHILDHOOD EDUCATION DEMONSTRATION PROJECT (51434-001) PROJECT PREPARATORY TECHNICAL ASSISTANCE TA 9589 - CHN CLIMATE RISK AND VULNERABILITY ASSESSMENT June 2020 2 Contents Page I. OVERVIEW 3 A. Background 3 B. Project Components 4 1. Climate Screening 5 2. Climatic Trends 7 3. Review of Literature Concerning Climatic Change 11 II. ASSESSING ADAPTATION NEEDS 13 A. Impact Assessment 13 B. Vulnerability Assessment 14 1. ECE Childcare Centers (Kindergartens) 14 2. Training and Career Center 14 C. Adaptation Analysis and Measures 14 1. Design and Performance strategies 15 2. Non-Engineering Measures 15 TABLES Table 1: Program Scope Table 2: Climate screening by ADB tool Table 3: Climate screening by literature review Table 4: Literature reviewed Table 5: Adaptation/Resilience Measures FIGURES Figure1: Annual mean temperature and precipitation anomaly series in Jiangxi province Figure2: Annual mean temperature projections under different RCPs Figure3: Statistics of projected temperature data from 2014 to 2020 Figure4: Precipitation projections under different RCPs Figure5: Statistics of projected precipitation data from 2014 to 2020 ANNEX Abstract of Feature Analysis of Weather Change in Recent 52 Years in Jiangxi Province 3 I. OVERVIEW A. Background 1. Climate Risk and Vulnerability Assessment is a part of the Final Report of Project Preparatory Technical Assistance (PPTA) for preparing finalizing Jiangxi Shangrao Early Childhood Education Demonstration Project (The project). The project is a cofinanced project initiated by Asian Development Bank (ADB) and the Government of Jiangxi Province. The project activities are dispersed in 12 counties and administration districts of Shangrao Municipality. -

Minimum Wage Standards in China August 11, 2020
Minimum Wage Standards in China August 11, 2020 Contents Heilongjiang ................................................................................................................................................. 3 Jilin ............................................................................................................................................................... 3 Liaoning ........................................................................................................................................................ 4 Inner Mongolia Autonomous Region ........................................................................................................... 7 Beijing......................................................................................................................................................... 10 Hebei ........................................................................................................................................................... 11 Henan .......................................................................................................................................................... 13 Shandong .................................................................................................................................................... 14 Shanxi ......................................................................................................................................................... 16 Shaanxi ...................................................................................................................................................... -

Download Article
Advances in Social Science, Education and Humanities Research (ASSEHR), volume 189 2018 International Conference on Management and Education, Humanities and Social Sciences (MEHSS 2018) Research on Reconstructing the Old Building Space in Inheritance of City Culture-Taking the Architecture of the Taoxichuan in Jingdezhen as an Example Han Wei School of Art and Design, Ceramic Institute, Jingdezhen 333000, China [email protected] Keywords: cultural heritage; transformation and upgrading. Abstract. There are many state-owned porcelain factories in Jingdezhen, which have made great contributions to the development and promotion of the ceramic culture. However, due to the influence of the market economy, and its own poor management, Jingdezhen porcelain factories gradually closed down to fall into disuse. Under the promotion of the creative culture industry, some old porcelain factories have been transformed to be used, but only limited to the creative industry model of the porcelain culture. Taking Taoxichuan cultural industrial park in Jingdezhen as an example, this paper analyzes the meaning of Jingdezhen post-industrial landscape regeneration and Jingdezhen industrial ruins problems and proposes the strategy of the industrial ruins renovation and landscape regeneration, so as to protect and continue Jingdezhen industrial heritage landscape culture, promote the development of various post-industrial landscape. We start with the transformation and upgrading of Taoxichuan, study and learn from the “Taoxichuan phenomenon”, and go to one road to rejuvenate the glorious dream of millennium porcelain capital. 1. The Taoxichuan Phenomenon Taoxichuan is located in the old industrial area of Jingdezhen City; there are a large number of ceramic industrial heritages and bears the heavy historical memory of the millennium porcelain culture in Jingdezhen.