Manuscript of MS Thesis
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

How Whales Used to Filter: Exceptionally Preserved Baleen in A
Journal of Anatomy J. Anat. (2017) 231, pp212--220 doi: 10.1111/joa.12622 How whales used to filter: exceptionally preserved baleen in a Miocene cetotheriid Felix G. Marx,1,2,3 Alberto Collareta,4,5 Anna Gioncada,4 Klaas Post,6 Olivier Lambert,3 Elena Bonaccorsi,4 Mario Urbina7 and Giovanni Bianucci4 1School of Biological Sciences, Monash University, Clayton, Vic., Australia 2Geosciences, Museum Victoria, Melbourne, Vic., Australia 3D.O. Terre et Histoire de la Vie, Institut Royal des Sciences Naturelles de Belgique, Brussels, Belgium 4Dipartimento di Scienze della Terra, Universita di Pisa, Pisa, Italy 5Dottorato Regionale in Scienze della Terra Pegaso, Pisa, Italy 6Natuurhistorisch Museum Rotterdam, Rotterdam, The Netherlands 7Departamento de Paleontologıa de Vertebrados, Museo de Historia Natural de la Universidad Nacional Mayor de San Marcos, Lima, Peru Abstract Baleen is a comb-like structure that enables mysticete whales to bulk feed on vast quantities of small prey, and ultimately allowed them to become the largest animals on Earth. Because baleen rarely fossilises, extremely little is known about its evolution, structure and function outside the living families. Here we describe, for the first time, the exceptionally preserved baleen apparatus of an entirely extinct mysticete morphotype: the Late Miocene cetotheriid, Piscobalaena nana, from the Pisco Formation of Peru. The baleen plates of P. nana are closely spaced and built around relatively dense, fine tubules, as in the enigmatic pygmy right whale, Caperea marginata. Phosphatisation of the intertubular horn, but not the tubules themselves, suggests in vivo intertubular calcification. The size of the rack matches the distribution of nutrient foramina on the palate, and implies the presence of an unusually large subrostral gap. -

PDF of Manuscript and Figures
The triple origin of whales DAVID PETERS Independent Researcher 311 Collinsville Avenue, Collinsville, IL 62234, USA [email protected] 314-323-7776 July 13, 2018 RH: PETERS—TRIPLE ORIGIN OF WHALES Keywords: Cetacea, Mysticeti, Odontoceti, Phylogenetic analyis ABSTRACT—Workers presume the traditional whale clade, Cetacea, is monophyletic when they support a hypothesis of relationships for baleen whales (Mysticeti) rooted on stem members of the toothed whale clade (Odontoceti). Here a wider gamut phylogenetic analysis recovers Archaeoceti + Odontoceti far apart from Mysticeti and right whales apart from other mysticetes. The three whale clades had semi-aquatic ancestors with four limbs. The clade Odontoceti arises from a lineage that includes archaeocetids, pakicetids, tenrecs, elephant shrews and anagalids: all predators. The clade Mysticeti arises from a lineage that includes desmostylians, anthracobunids, cambaytheres, hippos and mesonychids: none predators. Right whales are derived from a sister to Desmostylus. Other mysticetes arise from a sister to the RBCM specimen attributed to Behemotops. Basal mysticetes include Caperea (for right whales) and Miocaperea (for all other mysticetes). Cetotheres are not related to aetiocetids. Whales and hippos are not related to artiodactyls. Rather the artiodactyl-type ankle found in basal archaeocetes is also found in the tenrec/odontocete clade. Former mesonychids, Sinonyx and Andrewsarchus, nest close to tenrecs. These are novel observations and hypotheses of mammal interrelationships based on morphology and a wide gamut taxon list that includes relevant taxa that prior studies ignored. Here some taxa are tested together for the first time, so they nest together for the first time. INTRODUCTION Marx and Fordyce (2015) reported the genesis of the baleen whale clade (Mysticeti) extended back to Zygorhiza, Physeter and other toothed whales (Archaeoceti + Odontoceti). -

SMC 11 Gill 1.Pdf
SMITHSONIAN MISCELLANEOUS COLLECTIONS. 230 ARRANGEMENT FAMILIES OF MAMMALS. WITH ANALYTICAL TABLES. PREPARED FOR THE SMITHSONIAN INSTITUTION. BY THEODORE GILL, M.D., Ph.D. WASHINGTON: PUBLISHED BY THE SMITHSONIAN INSTITUTION. NOVEMBER, 1872. ADVERTISEMENT. The following list of families of Mammals, with analytical tables, has been prepared by Dr. Theodore Gill, at the request of the Smithsonian Institution, to serve as a basis for the arrangement of the collection of Mammals in the National Museum ; and as frequent applications for such a list have been received by the Institution, it has been thought advisable to publish it for more extended use. In provisionally adopting this system for the purpose mentioned, the Institution, in accordance with its custom, disclaims all responsibility for any of the hypothetical views upon which it may be based. JOSEPH HENRY, Secretary, S. I. Smithsonian Institution, Washington, October, 1872. (iii) CONTENTS. I. List of Families* (including references to synoptical tables) 1-27 Sub-Class (Eutheria) Placentalia s. Monodelpbia (1-121) 1, Super-Order Educabilia (1-73) Order 1. Primates (1-8) Sub-Order Anthropoidea (1-5) " Prosimiae (6-8) Order 2. Ferae (9-27) Sub-Order Fissipedia (9-24) . " Pinnipedia (25-27) Order 3. Ungulata (28-54) Sub-Order Artiodactyli (28-45) " Perissodactyli (46-54) Order 4. Toxodontia (55-56) . Order 5. Hyracoidea (57) Order 6. Proboscidea (58-59) Diverging (Educabilian) series. Order 7. Sirenia' (60-63) Order 8. Cete (64-73) . Sub-Order Zeuglodontia (64-65) " Denticete (66-71) . Mysticete (72-73) . Super-Order Ineducabilia (74-121) Order 9. Chiroptera (74-82) . Sub-Order Aniinalivora (74-81) " Frugivora (82) Order 10. -

The Taxonomic and Evolutionary History of Fossil and Modern Balaenopteroid Mysticetes
Journal of Mammalian Evolution, Vol. 12, Nos. 1/2, June 2005 (C 2005) DOI: 10.1007/s10914-005-6944-3 The Taxonomic and Evolutionary History of Fossil and Modern Balaenopteroid Mysticetes Thomas A. Demer´ e,´ 1,4 Annalisa Berta,2 and Michael R. McGowen2,3 Balaenopteroids (Balaenopteridae + Eschrichtiidae) are a diverse lineage of living mysticetes, with seven to ten species divided between three genera (Megaptera, Balaenoptera and Eschrichtius). Extant members of the Balaenopteridae (Balaenoptera and Megaptera) are characterized by their engulfment feeding behavior, which is associated with a number of unique cranial, mandibular, and soft anatomical characters. The Eschrichtiidae employ suction feeding, which is associated with arched rostra and short, coarse baleen. The recognition of these and other characters in fossil balaenopteroids, when viewed in a phylogenetic framework, provides a means for assessing the evolutionary history of this clade, including its origin and diversification. The earliest fossil balaenopterids include incomplete crania from the early late Miocene (7–10 Ma) of the North Pacific Ocean Basin. Our preliminary phylogenetic results indicate that the basal taxon, “Megaptera” miocaena should be reassigned to a new genus based on its possession of primitive and derived characters. The late late Miocene (5–7 Ma) balaenopterid record, except for Parabalaenoptera baulinensis and Balaenoptera siberi, is largely undescribed and consists of fossil specimens from the North and South Pacific and North Atlantic Ocean basins. The Pliocene record (2–5 Ma) is very diverse and consists of numerous named, but problematic, taxa from Italy and Belgium, as well as unnamed taxa from the North and South Pacific and eastern North Atlantic Ocean basins. -

The Biology of Marine Mammals
Romero, A. 2009. The Biology of Marine Mammals. The Biology of Marine Mammals Aldemaro Romero, Ph.D. Arkansas State University Jonesboro, AR 2009 2 INTRODUCTION Dear students, 3 Chapter 1 Introduction to Marine Mammals 1.1. Overture Humans have always been fascinated with marine mammals. These creatures have been the basis of mythical tales since Antiquity. For centuries naturalists classified them as fish. Today they are symbols of the environmental movement as well as the source of heated controversies: whether we are dealing with the clubbing pub seals in the Arctic or whaling by industrialized nations, marine mammals continue to be a hot issue in science, politics, economics, and ethics. But if we want to better understand these issues, we need to learn more about marine mammal biology. The problem is that, despite increased research efforts, only in the last two decades we have made significant progress in learning about these creatures. And yet, that knowledge is largely limited to a handful of species because they are either relatively easy to observe in nature or because they can be studied in captivity. Still, because of television documentaries, ‘coffee-table’ books, displays in many aquaria around the world, and a growing whale and dolphin watching industry, people believe that they have a certain familiarity with many species of marine mammals (for more on the relationship between humans and marine mammals such as whales, see Ellis 1991, Forestell 2002). As late as 2002, a new species of beaked whale was being reported (Delbout et al. 2002), in 2003 a new species of baleen whale was described (Wada et al. -

This Is a Postprint That Has Been Peer Reviewed and Published in Naturwissenschaften. the Final, Published Version of This Artic
This is a postprint that has been peer reviewed and published in Naturwissenschaften. The final, published version of this article is available online. Please check the final publication record for the latest revisions to this article. Boessenecker, R.W. 2013. Pleistocene survival of an archaic dwarf baleen whale (Mysticeti: Cetotheriidae). Naturwissenschaften 100:4:365-371. Pleistocene survival of an archaic dwarf baleen whale (Mysticeti: Cetotheriidae) Robert W. Boessenecker1,2 1Department of Geology, University of Otago, 360 Leith Walk, Dunedin, New Zealand 2 University of California Museum of Paleontology, University of California, Berkeley, California, U.S.A. *[email protected] Abstract Pliocene baleen whale assemblages are characterized by a mix of early records of extant mysticetes, extinct genera within modern families, and late surviving members of the extinct family Cetotheriidae. Although Pleistocene baleen whales are poorly known, thus far they include only fossils of extant genera, indicating late Pliocene extinctions of numerous mysticetes alongside other marine mammals. Here, a new fossil of the late Neogene cetotheriid mysticete Herpetocetus is reported from the Lower to Middle Pleistocene Falor Formation of Northern California. This find demonstrates that at least one archaic mysticete survived well into the Quaternary Period, indicating a recent loss of a unique niche and a more complex pattern of Plio-Pleistocene faunal overturn for marine mammals than has been previously acknowledged. This discovery also lends indirect support to the hypothesis that the pygmy right whale (Caperea marginata) is an extant cetotheriid, as it documents another cetotheriid nearly surviving to modern times. Keywords Cetacea; Mysticeti; Cetotheriidae; Pleistocene; California Introduction The four modern baleen whale (Cetacea: Mysticeti) families include fifteen gigantic filter-feeding species. -
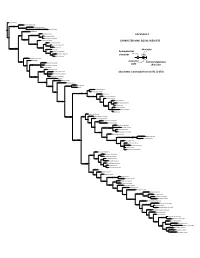
Appendices 2-6 As PDF for Download
Zygorhiza kochii 19 61 128148160 Archaeodelphis patrius 11 13 42 44 62 64 105159161 1 1 1 1 1 39 43 45 52 102150220 3 2 1 1 1 1 1 2 2 20 50 81 86 88 96 165 Waipatia maerewhenua 1 1 1 1 1 1 2 9 17 19 36 49 56 77 89 100103106107116120133145146151162163171196203210 1 2 1 1 1 1 4 Physeter catodon 1 3 2 1 2 1 1 1 1 1 1 1 2 1 1 1 1 1 3 1 1 2 1 2 27 41 46 102113148155188 ChM PV4745 0 1 1 1 1 1 1 1 23 26 Janjucetus hunderi 85 152 4 22 24 40 66 67 71 126213220 1 0 APPENDIX 2 1 1 2 1 1 2 0 1 1 0 1 2 34 188 Mammalodon colliveri 1 1 Mammalodon hakataramea 0 32 39 42 154161 101136 0 1 1 1 1 2 Morawanocetus yabukii 1 1 CHARACTER MAP, EQUAL WEIGHTS 71 136 Chonecetus sookensis 2 18 69 89 98 99 190228 100 1 1 1 1 1 1 1 1 1 2 0 110111 113 Chonecetus goedertorum 69 148238 28 33 78 220242 2 3 1 Fucaia buelli 3 38 54 57 76 100126134252 0 1 1 0 1 1 3 1 42 82 107111 145 1 1 1 1 1 1 1 1 1 OCPC 1178 0 0 1 1 2 character 97 0 0 Aetiocetus polydentatus 60 101106 1 homoplasious 73 126201 0 1 1 136 Aetiocetus cotylalveus 1 0 0 188 character 1 Aetiocetus weltoni 158 3 35 46 48 61 99 Llanocetus denticrenatus 0 1 1 1 1 4 39 71 OU GS10897 2 18 72 138 0 1 1 1 90 98 99 103129 character 1 1 1 1 Sitsqwayk cornisorum non homoplasious 0 1 1 1 2 119135145167170180181183184186187 109137268 13 113 state Tlaxcallicetus guaycurae character 5 1 3 1 1 1 1 1 2 1 1 1 1 1 1 1 205 Tlaxcallicetus sp. -

Piscivory in a Miocene Cetotheriidae of Peru: First Record of Fossilized Stomach Content for an Extinct Baleen-Bearing Whale
Piscivory in a Miocene Cetotheriidae of Peru: first record of fossilized stomach content for an extinct baleen-bearing whale Alberto Collareta1,2, *, Walter Landini1, Olivier Lambert3, Klaas Post4, Chiara Tinelli1, Claudio Di Celma5, Daniele Panetta6, Maria Tripodi6, Piero A. Salvadori6, Davide Caramella7, Damiano Marchi8,9, Mario Urbina10, Giovanni Bianucci1 1 Dipartimento di Scienze della Terra, Università di Pisa, via Santa Maria 53, 56126 Pisa, Italy 2 Dottorato Regionale in Scienze della Terra Pegaso, via S. Maria 53, 56126 Pisa, Italy 3 Institut royal des Sciences naturelles de Belgique, D.O. Terre et Histoire de la Vie, rue Vautier 29, 1000 Brussels, Belgium 4 Natuurhistorisch Museum Rotterdam, Westzeedijk 345, 3015 AA Rotterdam, The Netherlands. 5 Scuola di Scienze e Tecnologie, Università di Camerino, via Gentile III da Varano 1, 62032 Camerino, Italy 6 Istituto di Fisiologia Clinica, Consiglio Nazionale delle Ricerche, via G. Moruzzi 1, 56124 Pisa, Italy 7 Radiologia Diagnostica e Interventistica, Università di Pisa, via Roma 67, 56126 Pisa, Italy 8 Dipartimento di Biologia, Università di Pisa, via L. Ghini 13, 56126 Pisa, Italy 9 Evolutionary Studies Institute, University of the Witwatersrand, Private Bag 3, 2050 Wits, South Africa 10 Departamento de Paleontologia de Vertebrados, Museo de Historia Natural de la Universidad Nacional Mayor de San Marcos, Avenida Arenales 1256, Lima 14, Peru *Corresponding author. E-mail address: [email protected]. Telephone number: +39 346 3168173; +39 050 2215750. 1 Abstract Instead of teeth, modern mysticetes bear hair-fringed keratinous baleen plates that permit various bulk-filtering predation techniques (from subsurface skimming to lateral benthic suction and engulfment) devoted to various target prey (from small invertebrates to schooling fish). -

Universidad Nacional Mayor De San Marcos Universidad Del Perú
Universidad Nacional Mayor de San Marcos Universidad del Perú. Decana de América Facultad de Ciencias Biológicas Escuela Profesional de Ciencias Biológicas Anatomía craneana y posición filogenética de un nuevo cachalote enano (Odontoceti: Kogiidae) del mioceno tardío de la formación Pisco, Arequipa, Perú TESIS Para optar el Título Profesional de Biólogo con mención en Zoología AUTOR Aldo Marcelo BENITES PALOMINO ASESOR Víctor PACHECO TORRES Lima, Perú 2018 Dedicatoria Esta tesis va dedicada a todos aquellos niños y niñas que sintieron fascinación por los dinosaurios desde pequeños, para aquellos que se interesaron por conocer la naturaleza y que eligieron seguir el camino de ser científicos, como alguna vez lo hice algunos años atrás, para que sepan que no es un camino fácil. Pero que, si siguen pensando así y siguen fascinándose con cada pequeña pizca de naturaleza, se vuelve una de las travesías más asombrosas que puede haber. i Agradecimientos A mi asesor, Dr. Víctor Pacheco, por haberme introducido por primera vez al mundo de la sistemática, su apoyo, comentarios y correcciones durante la realización del presente trabajo. Al Dr. Niels Valencia por haber apoyado al laboratorio al que pertenezco desde el inicio y sus revisiones de este manuscrito. Al Dr. César Aguilar por sus comentarios de este manuscrito de tesis y discusiones de sistemática. A la Dra. Rina Ramírez por presidir el comité de sustentación y por sus comentarios sobre el manuscrito final. A mi mentor y co-asesor, Dr. Rodolfo Salas-Gismondi, por haberme apoyado a lo largo de toda mi trayectoria universitaria y haberse arriesgado al recibirme a tan temprana edad en el laboratorio que ahora considero mi hogar, el Departamento de Paleontología de Vertebrados. -

A Large Late Miocene Cetotheriid (Cetacea, Mysticeti) from the Netherlands Clarifies the Status of Tranatocetidae
A large Late Miocene cetotheriid (Cetacea, Mysticeti) from the Netherlands clarifies the status of Tranatocetidae Felix G. Marx1,2,3,4, Klaas Post5, Mark Bosselaers2,6 and Dirk K. Munsterman7 1 Department of Geology, Université de Liège, Liège, Belgium 2 Directorate of Earth and History of Life, Royal Belgian Institute of Natural Sciences, Brussels, Belgium 3 Palaeontology, Museums Victoria, Melbourne, Victoria, Australia 4 School of Biological Sciences, Monash University, Clayton, Victoria, Australia 5 Natuurhistorisch Museum, Rotterdam, The Netherlands 6 Zeeland Royal Society of Sciences, Middelburg, The Netherlands 7 Netherlands Institute of Applied Geoscience TNO - National Geological Survey, Utrecht, The Netherlands ABSTRACT Cetotheriidae are a group of small baleen whales (Mysticeti) that evolved alongside modern rorquals. They once enjoyed a nearly global distribution, but then largely went extinct during the Plio-Pleistocene. After languishing as a wastebasket taxon for more than a century, the concept of Cetotheriidae is now well established. Nevertheless, the clade remains notable for its variability, and its scope remains in flux. In particular, the recent referral of several traditional cetotheriids to a new and seemingly unrelated family, Tranatocetidae, has created major phylogenetic uncertainty. Here, we describe a new species of Tranatocetus, the type of Tranatocetidae, from the Late Miocene of the Netherlands. Tranatocetus maregermanicum sp. nov. clarifies several of the traits previously ascribed to this genus, and reveals distinctive auditory and mandibular morphologies suggesting cetotheriid affinities. This interpretation is supported by a large phylogenetic analysis, which mingles cetotheriids and tranatocetids within a unified clade. As a result, we suggest that both groups should be reintegrated into the single family Cetotheriidae. -
Did the Giant Extinct Shark Carcharocles Megalodon Target Small Prey? Bite Marks on Marine
1 Did the giant extinct shark Carcharocles megalodon target small prey? Bite marks on marine 2 mammal remains from the late Miocene of Peru 3 4 Alberto Collaretaa,b, Olivier Lambertc, Walter Landinia, Claudio Di Celmad, Elisa Malinvernoe, 5 Rafael Varas-Malcaf, Mario Urbinaf, Giovanni Bianuccia 6 7 a Dipartimento di Scienze della Terra, Università di Pisa, 56126 Pisa, Italy 8 b Dottorato Regionale in Scienze della Terra Pegaso, 56126 Pisa, Italy 9 c D.O. Terre et Histoire de la Vie, Institut Royal des Sciences Naturelles de Belgique, 1000 Brussels, 10 Belgium 11 d Scuola di Scienze e Tecnologie, Università di Camerino, 62032 Camerino, Italy 12 e Dipartimento di Scienze dell'Ambiente e del Territorio e di Scienze della Terra, Università di 13 Milano Bicocca, 20126 Milano, Italy 14 f Departamento de Paleontologia de Vertebrados, Museo de Historia Natural-UNMSM, Lima 14, 15 Peru 16 17 Author for correspondence: 18 Alberto Collareta. 19 E-mail address: [email protected] 20 ORCID ID: 0000-0002-6513-8882 1 21 Abstract 22 We report on bite marks incising fossil mammal bones collected from upper Miocene deposits of 23 the Pisco Formation exposed at Aguada de Lomas (southern Peru) and attributed to the giant 24 megatooth shark Carcharocles megalodon. The bitten material includes skull remains referred to 25 small-sized baleen whales as well as fragmentary cetacean and pinniped postcrania. These 26 occurrences, the first in their kind from the Southern Hemisphere, significantly expand the still 27 scarce record of bite marks for C. megalodon; moreover, for the first time a prey (or scavenging 28 item) of C. -

Burke from Home
BURKE FROM HOME WHALES WHAT’S INSIDE • Whales are mammals, just like us! Solve the evolution of whales puzzle to learn about the ancient relatives of modern whales. • Learn about different whale species and how they eat. • Want to take a deep dive? Check out more resources on our website. INTRODUCTION Whales live in every major ocean, from the freezing cold waters of the Arctic and Antarctic to the warm tropical waters near the equator. Whales travel, hunt, socialize, and even sing. Whales belong to a group of animals called cetaceans, which also includes dolphins and porpoises. There are about 90 known species of cetaceans. Have you ever seen a whale? Where did you see it? What was it doing? If you’ve never seen a whale, where could you go to see one? What could you bring with you to help find a whale? A good place to start is to learn more about whales and what they do. Let’s dive in! EVOLUTION OF WHALES TAKE A LOOK at this picture showing the bones in a human arm and the bones in a whale fin. What do you see? How are they similar? How are they different? HUMAN ARM BONES WHALE FIN BONES WHALES 1 | WHALES Can you feel the bones in your arm? There is one large bone from your shoulder to your elbow. From your elbow to your wrist, there are actually two bones that sit side-by-side. There are many small bones that make up your wrist and hand. The small narrow bones in your fingers are called phalanges.