Optimisation of a New Class of Peptide-Based MRI Contrast Agent
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

The Study Programme for the Quality Management of Essential Medicines - Good Manufacturing Practical (GMP) and Inspection
The Study Programme for the Quality Management of Essential Medicines - Good Manufacturing Practical (GMP) and Inspection - Country Reports Japan International Corporation of Welfare Services (JICWELS) Contents 1. Cambodia 1 2. Indonesia 70 3. Malaysia 91 4. Philippines 116 5. Sri Lanka 141 6. Thailand 161 The Study Programme for the Quality Management of Essential Medicines - Good Manufacturing Practical (GMP) and Inspection - Cambodia -1- KINGDOM OF CAMBODIA Nation Religion King Ministry of Health Department of Drugs and Food Country Report The Study Program on Quality Management of Essential Medicines Good Manufacturing Practice (GMP) and Inspection November 4, 2012 – November 30, 2012 Sponsored by : The Government of Japan Japan International Cooperation Agency (JICA) Department of Drugs and Food Ministry of Health, Cambodia. -2- I- COUNTRY PROFILE -3- A-Geography Cambodia is an agricultural country located in South East Asia which bordering the Gulf of Thailand, between Thailand, Vietnam, and Laos. Its approximate geographical coordinates are 13°N 105°E. Its 2,572 km border is split among Vietnam (1,228 km), Thailand (803 km) and Laos (541 km), as well as 443 km of coastline. Cambodia covers 181,035 square kilometers in the southwestern part of the Indochina, Cambodia lies completely within the tropics; its southernmost points are only slightly more than 10° above the equator. The country is bounded on the north by Thailand and by Laos, on the east and southeast by Vietnam, and on the west by the Gulf of Thailand and by Thailand. It consists of the Tonle Sap Basin and the Mekong Lowlands. To the southeast of this great basin is the Mekong Delta, which extends through Vietnam to the South China Sea. -

++449Guide to Radiology Procedures.Pdf
For Alexander and Olivia For Elsevier Content Strategist: Jeremy Bowes Content Development Specialist: Helen Leng Project Manager: Julie Taylor Designer/Design Direction: Miles Hitchen Illustration Manager: Jennifer Rose Illustrator: David Gardner and Antbits Ltd CHAPMAN AND NAKIELNY’S GUIDE TO RADIOLOGICAL PROCEDURES Edited by Nick Watson MBChB MRCP FRCR Consultant Radiologist, University Hospital of North Staffordshire, Stoke-on-Trent; Senior Lecturer, Keele University Medical School, Keele, UK SIXTH EDITION Edinburgh • London • New York • Oxford • Philadelphia • St Louis • Sydney • Toronto 2014 © 2014 Elsevier Ltd. All rights reserved. No part of this publication may be reproduced or transmitted in any form or by any means, electronic or mechanical, including photocopying, recording, or any information storage and retrieval system, without permission in writing from the publisher. Details on how to seek permission, further information about the publisher’s permissions policies and our arrangements with organizations such as the Copyright Clearance Center and the Copyright Licensing Agency, can be found at our website: www.elsevier.com/permissions. This book and the individual contributions contained in it are protected under copyright by the publisher (other than as may be noted herein). First edition 1981 Second edition 1986 Third edition 1993 Fourth edition 2001 Fifth edition 2009 Sixth edition 2014 ISBN 9780702051814 Ebook ISBN: 9780702054792 British Library Cataloguing in Publication Data A catalogue record for this book is available from the British Library Library of Congress Cataloging in Publication Data A catalog record for this book is available from the Library of Congress Notices Knowledge and best practice in this field are constantly changing. As new research and experience broaden our understanding, changes in research methods, professional practices, or medical treatment may become necessary. -

Drug-Induced Anaphylaxis in China: a 10 Year Retrospective Analysis of The
Int J Clin Pharm DOI 10.1007/s11096-017-0535-2 RESEARCH ARTICLE Drug‑induced anaphylaxis in China: a 10 year retrospective analysis of the Beijing Pharmacovigilance Database Ying Zhao1,2,3 · Shusen Sun4 · Xiaotong Li1,3 · Xiang Ma1 · Huilin Tang5 · Lulu Sun2 · Suodi Zhai1 · Tiansheng Wang1,3,6 Received: 9 May 2017 / Accepted: 19 September 2017 © The Author(s) 2017. This article is an open access publication Abstract Background Few studies on the causes of (50.1%), mucocutaneous (47.4%), and gastrointestinal symp- drug-induced anaphylaxis (DIA) in the hospital setting are toms (31.3%). A total of 249 diferent drugs were involved. available. Objective We aimed to use the Beijing Pharma- DIAs were mainly caused by antibiotics (39.3%), traditional covigilance Database (BPD) to identify the causes of DIA Chinese medicines (TCM) (11.9%), radiocontrast agents in Beijing, China. Setting Anaphylactic case reports from (11.9%), and antineoplastic agents (10.3%). Cephalospor- the BPD provided by the Beijing Center for Adverse Drug ins accounted for majority (34.5%) of antibiotic-induced Reaction Monitoring. Method DIA cases collected by the anaphylaxis, followed by fuoroquinolones (29.6%), beta- BPD from January 2004 to December 2014 were adjudi- lactam/beta-lactamase inhibitors (15.4%) and penicillins cated. Cases were analyzed for demographics, causative (7.9%). Blood products and biological agents (3.1%), and drugs and route of administration, and clinical signs and plasma substitutes (2.1%) were also important contributors outcomes. Main outcome measure Drugs implicated in DIAs to DIAs. Conclusion A variety of drug classes were impli- were identifed and the signs and symptoms of the DIA cases cated in DIAs. -
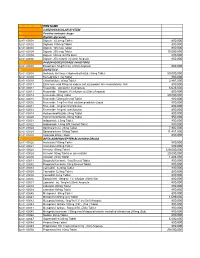
National Code Item Name 1
NATIONAL CODE ITEM NAME 1 CARDIOVASCULAR SYSTEM 1A Positive inotropic drugs 1AA Digtalis glycoside 02-01-00001 Digoxin 62.5mcg Tablet 800,000 02-01-00002 Digitoxin 100mcg Tablet 800,000 02-01-00003 Digoxin 125 mcg Tablet 800,000 02-01-00004 Digoxin 250 mcg Tablet 15,000,000 02-01-00005 Digoxin 50mcg /ml PG Elixir 800,000 02-01-00006 Digoxin 250 mcg/ml inj (2ml) Ampoule 800,000 1AB PHOSPHODIESTERASE INHIBITORS 02-01-00007 Enoximone 5mg/1ml inj (20ml) Ampoule 800,000 1B DIURETICS 02-01-00008 Amiloride Hcl 5mg + Hydrochlorthiazide 50mg Tablet 50,000,000 02-01-00009 Bumetanide 1 mg Tablet 800,000 02-01-00010 Chlorthalidone 50mg Tablet 2,867,000 02-01-00011 Ethacrynic acid 50mg as sodium salt inj (powder for reconstitution) Vial 800,000 02-01-00012 Frusemide 20mg/2ml inj Ampoule 6,625,000 02-01-00013 Frusemide 10mg/ml,I.V.infusion inj (25ml) Ampoule 800,000 02-01-00014 Frusemide 40mg Tablet 20,000,000 02-01-00015 Frusemide 500mg Scored Tablet 800,000 02-01-00016 Frusemide 1mg/1ml Oral solution peadiatric Liquid 800,000 02-01-00017 Frusemide 4mg/ml Oral Solution 800,000 02-01-00018 Frusemide 8mg/ml oral Solution 800,000 02-01-00019 Hydrochlorothiazide 25mg Tablet 800,000 02-01-00020 Hydrochlorothiazide 50mg Tablet 950,000 02-01-00021 Indapamide 2.5mg Tablet 800,000 02-01-00022 Indapamide 1.5mg S/R Coated Tablet 800,000 02-01-00023 Spironolactone 25mg Tablet 7,902,000 02-01-00024 Spironolactone 100mg Tablet 11,451,000 02-01-00025 Xipamide 20mg Tablet 800,000 1C BETA-ADRENOCEPTER BLOCKING DRUGS 02-01-00026 Acebutolol 100mg Tablet 800,000 02-01-00027 Acebutolol 200mg Tablet 800,000 02-01-00028 Atenolol 100mg Tablet 120,000,000 02-01-00029 Atenolol 50mg Tablet or (scored tab) 20,000,000 02-01-00030 Atenolol 25mg Tablet 1,483,000 02-01-00031 Bisoprolol fumarate 5mg Scored Tablet 800,000 02-01-00032 Bisoprolol fumarate 10mg Scored Tablet 800,000 02-01-00033 Carvedilol 6.25mg Tablet 800,000 02-01-00034 Carvedilol 12.5mg Tablet 800,000 02-01-00035 Carvedilol 25mg Tablet 800,000 02-01-00036 Esmolol Hcl 10mg/ml I.V. -

Page 1 Note: Within Nine Months from the Publication of the Mention
Europäisches Patentamt (19) European Patent Office & Office européen des brevets (11) EP 1 411 992 B1 (12) EUROPEAN PATENT SPECIFICATION (45) Date of publication and mention (51) Int Cl.: of the grant of the patent: A61K 49/04 (2006.01) A61K 49/18 (2006.01) 13.12.2006 Bulletin 2006/50 (86) International application number: (21) Application number: 02758379.8 PCT/EP2002/008183 (22) Date of filing: 23.07.2002 (87) International publication number: WO 2003/013616 (20.02.2003 Gazette 2003/08) (54) IONIC AND NON-IONIC RADIOGRAPHIC CONTRAST AGENTS FOR USE IN COMBINED X-RAY AND NUCLEAR MAGNETIC RESONANCE DIAGNOSTICS IONISCHES UND NICHT-IONISCHES RADIOGRAPHISCHES KONTRASTMITTEL ZUR VERWENDUNG IN DER KOMBINIERTEN ROENTGEN- UND KERNSPINTOMOGRAPHIEDIAGNOSTIK SUBSTANCES IONIQUES ET NON-IONIQUES DE CONTRASTE RADIOGRAPHIQUE UTILISEES POUR ETABLIR DES DIAGNOSTICS FAISANT APPEL AUX RAYONS X ET A L’IMAGERIE PAR RESONANCE MAGNETIQUE (84) Designated Contracting States: (74) Representative: Minoja, Fabrizio AT BE BG CH CY CZ DE DK EE ES FI FR GB GR Bianchetti Bracco Minoja S.r.l. IE IT LI LU MC NL PT SE SK TR Via Plinio, 63 20129 Milano (IT) (30) Priority: 03.08.2001 IT MI20011706 (56) References cited: (43) Date of publication of application: EP-A- 0 759 785 WO-A-00/75141 28.04.2004 Bulletin 2004/18 US-A- 5 648 536 (73) Proprietor: BRACCO IMAGING S.p.A. • K HERGAN, W. DORINGER, M. LÄNGLE W.OSER: 20134 Milano (IT) "Effects of iodinated contrast agents in MR imaging" EUROPEAN JOURNAL OF (72) Inventors: RADIOLOGY, vol. 21, 1995, pages 11-17, • AIME, Silvio XP002227102 20134 Milano (IT) • K.M. -

Magnetic Resonance Imaging (MRI) Contrast Agents Frequently Asked Questions
Magnetic Resonance Imaging (MRI) Contrast Agents Frequently Asked Questions 1. What is an MRI contrast agent? MRI contrast agents are chemical substances that contain the metal gadolinium. They are injected through a vein for many magnetic resonance imaging (MRI) scans. 2. What are the benefits of MRI contrast agents? When injected through a vein into the body, MRI contrast agents improve how internal organs, blood vessels, and other tissues look on the MRI images. This helps detect problems and diagnose medical conditions. For many medical conditions, the use of MRI contrast agents is a necessary part of the MRI. 3. What are the risks from MRI contrast agents? A. Injection problems. Any injection can cause injury to a nerve, artery, or vein, or result in infection. The contrast agent can also sometimes leak outside of the vein into the soft tissues during injection. This is called extravasation and is uncommon (about 1 in every 1,000 injections). This may be painful, but the pain is generally relieved by cold or warm compresses. B. Allergic reaction. Some people can be allergic to MRI contrast agents. There can sometimes be a mild reaction to the contrast agent such as itching and hives (about 1 in every 100 injections). Uncommonly, there can be a serious reaction to the contrast agent such as changes in blood pressure, cardiac distress, and respiratory distress (about 1 in every 10,000 injections). Very rarely this can result in death. For patients who have had a previous mild allergic reaction to an MRI contrast agent and need another MRI with MRI contrast agent, it is generally safe to do so after taking anti-allergy medications. -

Chemical Modifications of Porous Shape Memory Polymers For
molecules Article Chemical Modifications of Porous Shape Memory Polymers for Enhanced X-ray and MRI Visibility Grace K. Fletcher 1, Landon D. Nash 2, Lance M. Graul 1, Lindy K. Jang 1, Scott M. Herting 1, Matthew D. Wilcox 1, Tyler J. Touchet 1, Ana Katarina Sweatt 1, Mary P. McDougall 1,3, Steven M. Wright 1,3 and Duncan J. Maitland 1,2,* 1 Texas A&M University Biomedical Engineering, Bizzell St, College Station, TX 77843, USA; gracekfl[email protected] (G.K.F.); [email protected] (L.M.G.); [email protected] (L.K.J.); [email protected] (S.M.H.); [email protected] (M.D.W.); [email protected] (T.J.T.); [email protected] (A.K.S.); [email protected] (M.P.M.); [email protected] (S.M.W.) 2 Shape Memory Medical Inc., Santa Clara, CA 95054, USA; [email protected] 3 Texas A&M University Electrical and Computer Engineering, Bizzell St, College Station, TX 77843, USA * Correspondence: [email protected] Academic Editor: Irina Savina Received: 31 August 2020; Accepted: 12 October 2020; Published: 13 October 2020 Abstract: The goal of this work was to develop a shape memory polymer (SMP) foam with visibility under both X-ray and magnetic resonance imaging (MRI) modalities. A porous polymeric material with these properties is desirable in medical device development for applications requiring thermoresponsive tissue scaffolds with clinical imaging capabilities. Dual modality visibility was achieved by chemically incorporating monomers with X-ray visible iodine-motifs and MRI visible monomers with gadolinium content. Physical and thermomechanical characterization showed the effect of increased gadopentetic acid (GPA) on shape memory behavior. -

Sonovue® – Liver Dynamic Contrast Enhancement in Real Time
Bracco. The contrast imaging specialists. SonoVue® – Liver Dynamic Contrast Enhancement in Real Time BRACCO IMAGING Welcome to Bracco Imaging Bracco Imaging S.p.A., part of the Bracco Group, is one of the world’s leading companies in the diagnostic imaging business. Headquartered in Milan, Italy, Bracco Imaging develops, manufactures and markets diagnostic imaging agents and solutions that meet medical needs. Bracco Imaging offers a product and solution portfolio for all key diagnostic imaging modalities: X-Ray Imaging (including Computed Tomography-CT, Interventional Radiology, and Cardiac Catheterization), Magnetic Resonance Imaging (MRI), Contrast Enhanced Ultrasound (CEUS), Nuclear Medicine through radioactive tracers, and Gastrointestinal Endoscopy. The diagnostic imaging offer is completed by several medical devices and advanced administration systems for contrast imaging products in the fields of radiology and cardiology. These include the Empower line of contrast injection systems for CT and MRI procedures, the syringe-less contrast administration system CT Exprés and VueJect, our ultrasound contrast agents’ infusion pump. The Company operates in over 90 markets worldwide, either directly or indirectly, through subsidiaries, joint ventures, licenses and distribution partnership agreements. With an on-going research covering all key modalities, Bracco Imaging has a strong presence in key geographies: North America; Europe and Japan operating through the Joint Venture Bracco-Eisai Co., Ltd. The Company also operates in Brazil, South Korea, and China through the Joint Venture Bracco Sine Pharmaceutical Corp. Ltd. Operational investments have been made in order to achieve top quality and compliance with a sustainable eco-friendly production. Manufacturing activities are located in Italy, Switzerland, Japan, China and Germany. -

Gadolinium Based Contrast Agents
CE Credits Available CONSIDERATIONS IN THE SELECTION OF A NEW GADOLINIUM-BASED CONTRAST AGENT SUPPLEMENT TO MAY 2014 THE JOURNAL OF PRACTICAL MEDICAL IMAGING AND MANAGEMENT Considerations in the Selection of a New Gadolinium-Based Contrast Agent Michael F. Tweedle, PhD Emanuel Kanal, MD, FACR, FISMRM Robert Muller, PhD Stefanie Spielman Professor of Radiology Professor of Radiology and Neuroradiology Department of General, Organic & The Ohio State University University of Pittsburgh Medical Center Biochemical Chemistry Columbus, OH Pittsburgh, PA University of Mons Mons, Belgium Supported by an unrestricted educational grant from © May 2014 www.appliedradiology.com SUPPLEMENT TO APPLIED RADIOLOGY n 1 Publisher Kieran Anderson Executive Editor Cristen Bolan Art and Production Barbara A. Shopiro Applied Radiology and this supplement, Considerations in the Selection of a New Gadolinium-Based Contrast Agent, are published by Anderson Publishing, Ltd. The journal does not warrant the expertise of any author in a particular field, nor is it responsible for any statements by such authors. The opinions expressed in this supplement are those of the authors. They do not imply endorsement of advertised products and do not necessarily reflect the opinions or recommendations of our sponsors or the editors and staff of Applied Radiology. Copyright © 2014 by Anderson Publishing, Ltd., 180 Glenside Avenue, Scotch Plains, NJ 07076 All rights reserved. Cover images courtesy of Howard A. Rowley, MD. Considerations in the Selection of a New Gadolinium-Based Contrast Agent Our 3 esteemed faculty summarize the similarities and differences among the gadolinium-based contrast agents (GBCAs) currently utilized for magnetic resonance imaging (MRI), with emphasis on stability and relaxivity. -

Advisory Committee Briefing Document Medical Imaging Drugs Advisory Committee (MIDAC) September 8, 2017
Dotarem® (gadoterate meglumine) Injection – NDA# 204781 Advisory Committee Optimark® (gadoversetamide) Injection - NDAs# 020937, 020975 & 020976 Briefing Document Advisory Committee Briefing Document Medical Imaging Drugs Advisory Committee (MIDAC) September 8, 2017 DOTAREM® (gadoterate meglumine) Injection NDA 204781 Guerbet LLC, 821 Alexander Rd, Princeton, NJ 08540 OPTIMARK® (gadoversetamide) Injection NDAs 020937, 020975 & 020976 Liebel-Flarsheim Company LLC, 1034 Brentwood Blvd., Richmond Heights, MO 63117 ADVISORY COMMITTEE BRIEFING MATERIALS AVAILABLE FOR PUBLIC RELEASE Information provided within this briefing document is based upon medical and scientific information available to date. ADVISORY COMMITTEE BRIEFING MATERIALS AVAILABLE FOR PUBLIC RELEASE Page 1 / 168 Dotarem® (gadoterate meglumine) Injection – NDA# 204781 Advisory Committee Optimark® (gadoversetamide) Injection - NDAs# 020937, 020975 & 020976 Briefing Document EXECUTIVE SUMMARY Gadolinium-based contrast agents (GdCAs) are essential for use in magnetic resonance imaging (MRI). Although non-contrast-enhanced MRI may be sufficient for use in some clinical conditions, contrast-enhanced MRI (CE-MRI) using GdCA provides additional vital diagnostic information in a number of diseases. It is widely recognized that CE-MRI increases diagnostic accuracy and confidence, and thus can impact the medical and/or surgical management of patients. Based on the chemical structure of the complexing ligand, GdCA are classified as linear (L-GdCA) or macrocyclic (M-GdCA) and can be ionic or nonionic and those characteristics have a dramatic influence on the stability of the GdCA. Dotarem®, a M-GdCA, was first approved in France in 1989. US-FDA approval was obtained in March 2013 for “intravenous use with MRI of the brain (intracranial), spine and associated tissues in adult and pediatric patients (2 years of age and older) to detect and visualize areas with disruption of the blood brain barrier (BBB) and/or abnormal vascularity”, at the dose of 0.1 mmol/kg BW. -

PRESCRIBING INFORMATION CLARISCAN™ – Gadoteric Acid Please Refer to Full National Summary of Product Characteristics (Smpc) Before Prescribing
PRESCRIBING INFORMATION CLARISCAN™ – gadoteric acid Please refer to full national Summary of Product Characteristics (SmPC) before prescribing. Further information available on request. PRESENTATION Clariscan 0.5 mmol/mL solution for injection. Solution for injection containing 279.3 mg/ml gadoteric acid (as gadoterate meglumine) equivalent to 0.5 mmol/mL. INDICATIONS For diagnostic use only. Clariscan should be used only when diagnostic information is essential and not available with unenhanced magnetic resonance imaging (MRI). Contrast agent for contrast enhancement in MRI for a better visualisation/delineation. Adult and paediatric population (0-18 years): lesions of the brain, spine, and surrounding tissues. Adults and children over 6 months Whole body MRI. Non-coronary angiography in adults only. DOSAGE AND METHOD OF ADMINISTRATION This medicinal product should only be administered by trained healthcare professionals with technical expertise in performing and interpreting gadolinium enhanced MRI. The lowest dose that provides sufficient enhancement for diagnostic purposes should be used. The dose should be calculated based on the patient’s body weight, and should not exceed the recommended dose per kilogram of body weight detailed in this section. MRI of brain and spine: Adults: The recommended dose is 0.1 mmol/kg BW, i.e. 0.2 mL/kg BW. In patients with brain tumours, an additional dose of 0.2 mmol/kg BW, i.e. 0.4 mL/kg BW, may improve tumor characterisation and facilitate therapeutic decision making. Children (0-18 years): The recommended and maximum dose of Clariscan is 0.1 mmol/kg body weight. Do not use more than one dose during a scan. -

A Novel Anti-SARS-Cov2 Agent Targeting Viral Main Protease 3Clpro Demonstrated by Virtual Screening and in Vitro Assays
Bisindolylmaleimide IX: A novel anti-SARS-CoV2 agent targeting viral main protease 3CLpro demonstrated by virtual screening and in vitro assays Yash Gupta Loyola University Chicago Stritch School of Medicine, Chicago, IL, 60153, USA & Department of Medicine, Loyola University Medical Center, Chicago, IL, 60153, USA Dawid Maciorowski Loyola University Chicago Stritch School of Medicine, Chicago, IL, 60153, USA & Department of Medicine, Loyola University Medical Center, Chicago, IL, 60153, USA Samantha E. Zak United States Army Medical Research Institute of Infectious Diseases Krysten A. Jones Department of Chemistry, The University of Chicago, 5801 South Ellis Avenue, Chicago, Illinois, USA Rahul S. Kathayat Department of Chemistry, The University of Chicago, 5801 South Ellis Avenue, Chicago, Illinois, USA Saara-Anne Azizi Department of Chemistry, The University of Chicago, 5801 South Ellis Avenue, Chicago, Illinois, USA Raman Mathur Loyola University Chicago Stritch School of Medicine, Chicago, IL, 60153, USA & Department of Medicine, Loyola University Medical Center, Chicago, IL, 60153, USA Catherine M. Pearce Loyola University Chicago Stritch School of Medicine, Chicago, IL, 60153, USA & Department of Medicine, Loyola University Medical Center, Chicago, IL, 60153, USA David J. Ilc Loyola University Chicago Stritch School of Medicine, Chicago, IL, 60153, USA Hamza Husein Loyola University Chicago Stritch School of Medicine, Chicago, IL, 60153, USA Andrew S. Herbert United States Army Medical Research Institute of Infectious Diseases, Fort Detrick, MD, USA Ajay Bharti Division of Infectious Diseases, Department of Medicine, University of California, San Diego, CA, 92093, USA Brijesh Rathi Laboratory for Translational Chemistry and Drug Discovery, Hansraj College, University of Delhi, India Ravi Durvasula Loyola University Chicago Stritch School of Medicine, Chicago, IL, 60153, USA & Department of Medicine, Loyola University Medical Center, Chicago, IL, 60153, USA Daniel P.