Cause and Consequence of Recurrent Early Jurassic Anoxia Following The
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Cambrian Phytoplankton of the Brunovistulicum – Taxonomy and Biostratigraphy
MONIKA JACHOWICZ-ZDANOWSKA Cambrian phytoplankton of the Brunovistulicum – taxonomy and biostratigraphy Polish Geological Institute Special Papers,28 WARSZAWA 2013 CONTENTS Introduction...........................................................6 Geological setting and lithostratigraphy.............................................8 Summary of Cambrian chronostratigraphy and acritarch biostratigraphy ...........................13 Review of previous palynological studies ...........................................17 Applied techniques and material studied............................................18 Biostratigraphy ........................................................23 BAMA I – Pulvinosphaeridium antiquum–Pseudotasmanites Assemblage Zone ....................25 BAMA II – Asteridium tornatum–Comasphaeridium velvetum Assemblage Zone ...................27 BAMA III – Ichnosphaera flexuosa–Comasphaeridium molliculum Assemblage Zone – Acme Zone .........30 BAMA IV – Skiagia–Eklundia campanula Assemblage Zone ..............................39 BAMA V – Skiagia–Eklundia varia Assemblage Zone .................................39 BAMA VI – Volkovia dentifera–Liepaina plana Assemblage Zone (Moczyd³owska, 1991) ..............40 BAMA VII – Ammonidium bellulum–Ammonidium notatum Assemblage Zone ....................40 BAMA VIII – Turrisphaeridium semireticulatum Assemblage Zone – Acme Zone...................41 BAMA IX – Adara alea–Multiplicisphaeridium llynense Assemblage Zone – Acme Zone...............42 Regional significance of the biostratigraphic -

Stuttgarter Beiträge Zur Naturkunde
ZOBODAT - www.zobodat.at Zoologisch-Botanische Datenbank/Zoological-Botanical Database Digitale Literatur/Digital Literature Zeitschrift/Journal: Stuttgarter Beiträge Naturkunde Serie B [Paläontologie] Jahr/Year: 1977 Band/Volume: 26_B Autor(en)/Author(s): Ziegler Bernhard Artikel/Article: The "White" (Upper) Jurassic in Southern Germany 1- 79 5T(J 71^^-7 © Biodiversity Heritage Library, http://www.biodiversitylibrary.org/; www.zobodat.at Stuttgarter Beiträge zur Naturkunde Herausgegeben vom Staatlichen Museum für Naturkunde in Stuttgart Serie B (Geologie und Paläontologie), Nr. 26 .,.wr-..^ns > Stuttgart 1977 APR 1 U 19/0 The "White" (Upper) Jurassic D in Southern Germany By Bernhard Ziegler, Stuttgart With 11 plates and 42 figures 1. Introduction The Upper part of the Jurassic sequence in southern Germany is named the "White Jurassic" due to the light colour of its rocks. It does not correspond exactly to the Upper Jurassic as defined by the International Colloquium on the Jurassic System in Luxembourg (1962), because the lower Oxfordian is included in the "Brown Jurassic", and because the upper Tithonian is missing. The White Jurassic covers more than 10 000 square kilometers between the upper Main river near Staffelstein in northern Bavaria and the Swiss border west of the lake of Konstanz. It builds up the Swabian and the Franconian Alb. Because the Upper Jurassic consists mainly of light limestones and calcareous marls which are more resistent to the erosion than the clays and marls of the underlying Brown (middle) Jurassic it forms a steep escarpment directed to the west and northwest. To the south the White Jurassic dips below the Tertiary beds of the Molasse trough. -

Parish Plans Biodiversity Project
Parish Biodiversity Audit for Beer Consultation draft – April 2010 Anne Harvey Report commissioned by Devon County Council Data supplied by the Devon Biodiversity Records Centre Contents INTRODUCTION ..................................................................................................................................... 4 DESIGNATED SITES .............................................................................................................................. 6 SITES OF SPECIAL SCIENTIFIC INTEREST ............................................................................................ 6 Sidmouth to Beer Coast SSSI ...................................................................................................... 6 Beer Quarry and Caves SSSI ...................................................................................................... 9 SPECIAL AREAS OF CONSERVATION .................................................................................................. 10 Sidmouth to West Bay Special Area of Conservation ............................................................ 10 Beer Quarry and Caves Special Area of Conservation .......................................................... 10 Poole Bay to Lyme Bay Reefs draft Special Area of Conservation ...................................... 11 COUNTY WILDLIFE SITES ................................................................................................................... 11 Beer Quarry and Caves County Wildlife Site .......................................................................... -

Dorset and East Devon Coast for Inclusion in the World Heritage List
Nomination of the Dorset and East Devon Coast for inclusion in the World Heritage List © Dorset County Council 2000 Dorset County Council, Devon County Council and the Dorset Coast Forum June 2000 Published by Dorset County Council on behalf of Dorset County Council, Devon County Council and the Dorset Coast Forum. Publication of this nomination has been supported by English Nature and the Countryside Agency, and has been advised by the Joint Nature Conservation Committee and the British Geological Survey. Maps reproduced from Ordnance Survey maps with the permission of the Controller of HMSO. © Crown Copyright. All rights reserved. Licence Number: LA 076 570. Maps and diagrams reproduced/derived from British Geological Survey material with the permission of the British Geological Survey. © NERC. All rights reserved. Permit Number: IPR/4-2. Design and production by Sillson Communications +44 (0)1929 552233. Cover: Duria antiquior (A more ancient Dorset) by Henry De la Beche, c. 1830. The first published reconstruction of a past environment, based on the Lower Jurassic rocks and fossils of the Dorset and East Devon Coast. © Dorset County Council 2000 In April 1999 the Government announced that the Dorset and East Devon Coast would be one of the twenty-five cultural and natural sites to be included on the United Kingdom’s new Tentative List of sites for future nomination for World Heritage status. Eighteen sites from the United Kingdom and its Overseas Territories have already been inscribed on the World Heritage List, although only two other natural sites within the UK, St Kilda and the Giant’s Causeway, have been granted this status to date. -

Wessex Branch Newsletter
The Open University Geological Society Wessex Branch Newsletter Website http://ougs.org/wessex December 2015 CONTENTS Branch Organiser’s Letter Page 1 Season’s Greetings Lyme Regis fossiling, 13 Sept 2015 Pages 2-3 to all readers Anglesey, 23-25 September 2015 Pages 4-9 Hope to see you at our AGM and Rock of Ages, a note by Tony Cross Page 9 day of lectures in Wool on Minerals guide no. 17 – Musocovite Page 10 Saturday, 23 January 2016. Wessex Branch committee Page 10 OUGS Symposium 2016, Exeter Page 11 Other organisations’ events Page 12 2016 AGM notice and programme Page 13 Branch Organiser’s Letter Forthcoming Wessex Branch events Page 13 Dear All OUGS events listing Page 14 I hope you have enjoyed your geology over the past 12 months. Do look later in the newsletter many of them the only concrete involvement for future events and in particular the advert for they have with the branch, we have decided to the AGM and lecture Day on 23rd January 2016 continue to do so. The cost of printing and in Wool near Wareham (page 13) and the postage is just covered by our branch grant. th th Symposium in Exeter 8 to 11 July 2016 Our branch over the last 20 years has ended up (page 11). Thanks very much for the offers of with a surplus in the accounts so we are help with the symposium. The cut-off date for sponsoring the cost of the boat along the the symposium is much earlier than in previous Heritage Coast for the symposium in 2016 as years due to our contract with Exeter University well as paying for one of our very supportive so book early to ensure you are not field trip leaders, Alan Holiday. -
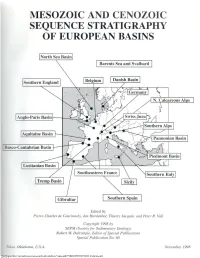
Mesozoic and Cenozoic Sequence Stratigraphy of European Basins
Downloaded from http://pubs.geoscienceworld.org/books/book/chapter-pdf/3789969/9781565760936_frontmatter.pdf by guest on 26 September 2021 Downloaded from http://pubs.geoscienceworld.org/books/book/chapter-pdf/3789969/9781565760936_frontmatter.pdf by guest on 26 September 2021 MESOZOIC AND CENOZOIC SEQUENCE STRATIGRAPHY OF EUROPEAN BASINS PREFACE Concepts of seismic and sequence stratigraphy as outlined in To further stress the importance of well-calibrated chronos- publications since 1977 made a substantial impact on sedimen- tratigraphic frameworks for the stratigraphic positioning of geo- tary geology. The notion that changes in relative sea level shape logic events such as depositional sequence boundaries in a va- sediment in predictable packages across the planet was intui- riety of depositional settings in a large number of basins, the tively attractive to many sedimentologists and stratigraphers. project sponsored a biostratigraphic calibration effort directed The initial stratigraphic record of Mesozoic and Cenozoic dep- at all biostratigraphic disciplines willing to participate. The re- ositional sequences, laid down in response to changes in relative sults of this biostratigraphic calibration effort are summarized sea level, published in Science in 1987 was greeted with great, on eight charts included in this volume. albeit mixed, interest. The concept of sequence stratigraphy re- This volume also addresses the question of cyclicity as a ceived much acclaim whereas the chronostratigraphic record of function of the interaction between tectonics, eustasy, sediment Mesozoic and Cenozoic sequences suffered from a perceived supply and depositional setting. An attempt was made to estab- absence of biostratigraphic and outcrop documentation. The lish a hierarchy of higher order eustatic cycles superimposed Mesozoic and Cenozoic Sequence Stratigraphy of European on lower-order tectono-eustatic cycles. -

Proceedings of the Ussher Society
Proceedings of the Ussher Society Research into the geology and geomorphology of south-west England Volume 6 Part 3 1986 Edited by G.M Power The Ussher Society Objects: To promote research into the geology and geomorphology of south- west England and the surrounding marine areas; to hold Annual Conferences at various places in South West England where those engaged in this research can meet formally to hear original contributions and progress reports and informally to effect personal contacts; to publish, proceedings of such Conferences or any other work which the Officers of the Society may deem suitable. Officers: Chairman Dr. C.T. Scrutton Vice-Chairman Dr. E. B. Selwood Secretary Mr M.C. George Treasurer Mr R.C. Scrivener Editor Dr. G.M. Power Committee Members Dr G. Warrington Mr. C. R. Morey Mr. C.D.N. Tubb Mr. C. Cornford Mr D. Tucker Membership of the Ussher Society is open to all on written application to the Secretary and payment of the subscription due on January lst each year. Back numbers may be purchased from the Secretary to whom correspondence should be directed at the following address: Mr M. C. George, Department of Geology, University of Exeter, North Park Road, Exeter, Devon EX4 4QE Proceedings of the Ussher Society Volume 6 Part 3 1986 Edited by G.M. Power Crediton, 1986 © Ussher Society ISSN 0566-3954 1986 Typeset, printed and bound bv Phillips & Co., The Kyrtonia Press, 115 High Street, Crediton, Devon EXl73LG Set in Baskerville and Printed by Photolithography Proceedings of the Ussher Society Volume 6, Part 3, 1986 Papers D.L. -

Your Free Independent Guide to Lyme Regis
your free independent guide to Lyme Regis @JURASSICMAGS jurassiccoastmagazine.co.uk It’s been a long journey. Excitement builds as you see Lyme in the distance. Take a heading of 284° and follow the leading light into the harbour. The light turns from red to white, you know you’re home. It’s time for a pint. Loaded with 6 different hops including Mosaic and Citra, 284° makes for a refreshing welcome to Lyme Regis. HOPPY LANDINGS WELCOME TO jurassiccoastmagazine.co.uk Evolution Since we set sail in 2014 with our pilot edition of Lyme Magazine, we have noticed one very common theme in our content. Evolution. Lyme Regis is a town which boasts centuries of history, and is situated on a unique coastline which displays millions of years of adaptation. But even today, over the last 6 years, we have seen great change in our little town. A new sea defence scheme, a wonderful new museum, some fantastic new eateries, an eclectic mix of artisans and world class events... just a few of the many designed in Lyme Regis by wonderful attractions that make Lyme one of the UK’s best coastal destinations. //coastline.agency It is because of these wonderful, ever changing highlights that we can keep bringing you Lyme Magazine. Whilst every care has been taken to ensure that Our aim is simple. Help promote businesses in and around Lyme Regis, and to tell the content of this publication is accurate, Coastline the story of the wonderful folk who call ‘The Pearl of Dorset’ home. We do this Publishing Ltd accepts no liability to any party for loss by providing visitors to The Jurassic Coast with a handbag-sized comprehensive or damage caused by errors or omissions. -

Parish Plans Biodiversity Project
Parish Biodiversity Audit for Axmouth Consultation draft – April 2010 Anne Harvey Report commissioned by Devon County Council Data supplied by the Devon Biodiversity Records Centre Contents INTRODUCTION ..................................................................................................................................... 4 DESIGNATED SITES .............................................................................................................................. 7 SITES OF SPECIAL SCIENTIFIC INTEREST ............................................................................................ 7 Axmouth to Lyme Regis Undercliffs SSSI .................................................................................. 7 Springhead Axmouth SSSI ......................................................................................................... 11 River Axe SSSI............................................................................................................................. 12 SPECIAL AREAS OF CONSERVATION .................................................................................................. 13 Sidmouth to West Bay Special Area of Conservation ............................................................ 13 River Axe Special Area of Conservation .................................................................................. 14 Poole Bay to Lyme Bay Reefs draft Special Area of Conservation ...................................... 14 NATIONAL NATURE RESERVES ......................................................................................................... -

Wessex Basin of Southern England - Field Trip Guide
CENTRAL & NORTH ATLANTIC CONJUGATE MARGINS CONFERENCE DUBLIN 2012 THIRD CONJUGATE MARGINS CONFERENCE 2012 Basin development and hydrocarbon systems of the Mesozoic and Cenozoic THE WESSEX BASIN OF SOUTHERN ENGLAND - FIELD TRIP GUIDE www.conjugatemargins.ie Third Conjugate Margins Conference‐ DUBLIN 2012 The Wessex Basin of Southern England‐ Field Trip guide Basin Development & Hydrocarbon systems of the Mesozoic and Cenozoic Leaders: Professor Grant Wach, Department of Earth Sciences, Dalhousie University, Halifax, Canada Grant Wach was appointed Professor of Petroleum Geoscience at Dalhousie University in 2002. A specialist in Reservoir Characterization and Stratigraphy, his interest in complex oil reservoirs began at Syncrude, in the oil sands of Alberta. Prior to Dalhousie he was with Exxon (now ExxonMobil) and was Geoscience Research Associate at Texaco (now Chevron) involved with exploration and commercialization with business units around the globe. He has conducted research on, and led field schools and courses in exploration, development and reservoir characterization in several countries. In 2012 he was the first recipient of “Professor of the Year” award from the AAPG Foundation. Professor Wach has an Honours B.A.(Geography/Geology) from the University of Western Ontario, a M.Sc. (Geology) from South Carolina, and a D.Phil. (Geology) from the University of Oxford. He can be contacted at +1‐902‐494‐2358; [email protected]. Professor Stephen Hesselbo, Department of Earth Sciences, University of Oxford and St. Peter’s College, -

Plesiosaurs (Reptilia; Sauropterygia) from the Braunjura (Middle Jurassic; Late Aalenian) of Southern Germany 51-62 ©Staatl
ZOBODAT - www.zobodat.at Zoologisch-Botanische Datenbank/Zoological-Botanical Database Digitale Literatur/Digital Literature Zeitschrift/Journal: Carolinea - Beiträge zur naturkundlichen Forschung in Südwestdeutschland Jahr/Year: 2004 Band/Volume: 62 Autor(en)/Author(s): Buchy Marie-Céline Artikel/Article: Plesiosaurs (Reptilia; Sauropterygia) from the Braunjura (Middle Jurassic; late Aalenian) of southern Germany 51-62 ©Staatl. Mus. f. Naturkde Karlsruhe & Naturwiss. Ver. Karlsruhe e.V.; download unter www.zobodat.at carolinea, 62 (2004): 51-62, 6 Abb.; Karlsruhe, 15.12.2004 51 iVIARIE-CELINh BUCHY Plesiosaurs (Reptilia; Sauropterygia) from the Braunjura (3 (Middle Jurassic; late Aalenian) of southern Germany Kurzfassung Introduction Plesiosauria (Reptilia; Sauropterygia) aus dem Braunjura ß (Mittlers Jura, Spätes Aalenian) Süddeutschlands. When the collections of the Geologisches Institut Obwohl Meeresreptilien aus dem Braunen Jura ß seit dem (Geological Institute) of the University of Freiburg were 19. Jahrhundert bekannt sind und in deutschen Sammlungen gelagert werden, wurden sie bisher nie detailliert beschrieben. dispersed in the 1980s, part of the vertebrate teaching In diesen Schichten kommen Plesiosaurier-, Thalattosuchier- collections was transferred to the Staatliches Museum und selten auch Ichthyosaurierreste vor. Sie sind alle als Frag für Naturkunde Karlsruhe (SMNK). The original labels mente erhalten, wahrscheinlich aufgrund eines flachmarinen, are now the only available data about these specimens hochenergetischen Ablagerungsraums. Die Cervicalwirbel aus (Munk , pers. comm.). den Sammlungen des Staatlichen Museums für Naturkunde The specimens described herein are part of the materi Karlsruhe, welche hier beschrieben werden sind die erste al transferred to the SMNK. According to the original la Nachweis elasmosaurider Plesiosaurier aus dem deutschen Dogger. bels they come from the ‘Braun Jura ß, Murchisonae Ei- senoolith, Wasseralfingen, Württemberg’, a unit dated Abstract to the late Aalenian (e.g. -

Sidmouth Lifeboat Pg26
2015 Part of the Jurassic Coast Magazine Series PICK ME UP! Saving Lives Sidmouth Lifeboat Pg26 EVENTS Pg20 GIFT GUIDE Fun Pg32 Pg29 for the kids SHARE YOUR THOUGHTS ^ Jacob’s Ladder 2015 @JurassicMags FOOD • CULTURE • ARTS • SHOPPING • EVENTS #JurassicMags Try the Chef’s specials at Fresh Fish Daily Being by the sea, there’s lots of fi sh on our menu all year round and Chef loves to create delicious, affordable specials on a daily basis from fresh ingredients delivered to our kitchens each day by our trusted local suppliers. With seasonal, locally sourced ingredients and fantastic views over Lyme Bay and Cobb Harbour, where else would you go for your daily fi sh (and chips!)? Tel. 01297 442668 www.bythebay.co.uk Follow us on facebook and twitter view online at jurassiccoastmagazine.co.uk Sidmouth Visitor 2015 discover the soul inside: of Sidmouth A week in... | 6 Five ideas for what to do during your stay in Sidmouth Welcome to the first-ever issue of Sidmouth Visitor, your free guide to everything the exquisite Seeing Stars | 10 Regency resort of Sidmouth has to offer. Discovery the link between Sidmouth and astronomy Sidmouth is a lovely seaside escape whether you’re visiting for the first time or ‘All aboard’ | 12 returning once again, this seaside town has something to offer everyone, with Why not see Sidmouth from its beautiful gardens, rich history and internationally acclaimed Folk Festival. an alternative view... We hope you enjoy this comprehensive guide to the best places to shop, dine, relax and explore whilst visiting this ‘Gateway to the Jurassic Coast’.