Cortical Re-Organization After Traumatic Brain Injury Elicited
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Meguro Walking Map
Meguro Walking Map Meguro Walking Map Primary print number No. 31-30 Published February 2, 2020 December 6, 2019 Published by Meguro City Edited by Health Promotion Section, Health Promotion Department; Sports Promotion Section, Culture and Sports Department, Meguro City 2-19-15 Kamimeguro, Meguro City, Tokyo Phone 03-3715-1111 Cooperation provided by Meguro Walking Association Produced by Chuo Geomatics Co., Ltd. Meguro City Total Area Course Map Contents Walking Course 7 Meguro Walking Courses Meguro Walking Course Higashi-Kitazawa Sta. Total Area Course Map C2 Walking 7 Meguro Walking Courses P2 Course 1: Meguro-dori Ave. Ikenoue Sta. Ke Walk dazzling Meguro-dori Ave. P3 io Inok Map ashira Line Komaba-todaimae Sta. Course 2: Komaba/Aobadai area Shinsen Sta. Walk the ties between Meguro and Fuji P7 0 100 500 1,000m Awas hima-dori St. 3 Course 3: Kakinokizaka/Higashigaoka area Kyuyamate-dori Ave. Walk the 1964 Tokyo Olympics P11 2 Komaba/Aobadai area Walk the ties between Meguro and Fuji Shibuya City Tamagawa-dori Ave. Course 4: Himon-ya/Meguro-honcho area Ikejiri-ohashi Sta. Meguro/Shimomeguro area Walk among the history and greenery of Himon-ya P15 5 Walk among Edo period townscape Daikan-yama Sta. Course 5: Meguro/Shimomeguro area Tokyu Den-en-toshi Line Walk among Edo period townscape P19 Ebisu Sta. kyo Me e To tro Hibiya Lin Course 6: Yakumo/Midorigaoka area Naka-meguro Sta. J R Walk a green road born from a culvert P23 Y Yutenji/Chuo-cho area a m 7 Yamate-dori Ave. a Walk Yutenji and the vestiges of the old horse track n o Course 7: Yutenji/Chuo-cho area t e L Meguro City Office i Walk Yutenji and the vestiges of the old horse track n P27 e / S 2 a i k Minato e y Kakinokizaka/Higashigaoka area o in City Small efforts, L Yutenji Sta. -
Introducing Tokyo Page 10 Panorama Views
Introducing Tokyo page 10 Panorama views: Tokyo from above 10 A Wonderful Catastrophe Ulf Meyer 34 The Informational World City Botond Bognar 42 Bunkyo-ku page 50 001 Saint Mary's Cathedral Kenzo Tange 002 Memorial Park for the Tokyo War Dead Takefumi Aida 003 Century Tower Norman Foster 004 Tokyo Dome Nikken Sekkei/Takenaka Corporation 005 Headquarters Building of the University of Tokyo Kenzo Tange 006 Technica House Takenaka Corporation 007 Tokyo Dome Hotel Kenzo Tange Chiyoda-ku page 56 008 DN Tower 21 Kevin Roche/John Dinkebo 009 Grand Prince Hotel Akasaka Kenzo Tange 010 Metro Tour/Edoken Office Building Atsushi Kitagawara 011 Athénée Français Takamasa Yoshizaka 012 National Theatre Hiroyuki Iwamoto 013 Imperial Theatre Yoshiro Taniguchi/Mitsubishi Architectural Office 014 National Showa Memorial Museum/Showa-kan Kiyonori Kikutake 015 Tokyo Marine and Fire Insurance Company Building Kunio Maekawa 016 Wacoal Building Kisho Kurokawa 017 Pacific Century Place Nikken Sekkei 018 National Museum for Modern Art Yoshiro Taniguchi 019 National Diet Library and Annex Kunio Maekawa 020 Mizuho Corporate Bank Building Togo Murano 021 AKS Building Takenaka Corporation 022 Nippon Budokan Mamoru Yamada 023 Nikken Sekkei Tokyo Building Nikken Sekkei 024 Koizumi Building Peter Eisenman/Kojiro Kitayama 025 Supreme Court Shinichi Okada 026 Iidabashi Subway Station Makoto Sei Watanabe 027 Mizuho Bank Head Office Building Yoshinobu Ashihara 028 Tokyo Sankei Building Takenaka Corporation 029 Palace Side Building Nikken Sekkei 030 Nissei Theatre and Administration Building for the Nihon Seimei-Insurance Co. Murano & Mori 031 55 Building, Hosei University Hiroshi Oe 032 Kasumigaseki Building Yamashita Sekkei 033 Mitsui Marine and Fire Insurance Building Nikken Sekkei 034 Tajima Building Michael Graves Bibliografische Informationen digitalisiert durch http://d-nb.info/1010431374 Chuo-ku page 74 035 Louis Vuitton Ginza Namiki Store Jun Aoki 036 Gucci Ginza James Carpenter 037 Daigaku Megane Building Atsushi Kitagawara 038 Yaesu Bookshop Kajima Design 039 The Japan P.E.N. -
Railway Lines in Tokyo and Its Suburbs
Minami-Sakurai Hasuta Shin-Shiraoka Fujino-Ushijima Shimizu-Koen Railway Lines in Tokyo and its Suburbs Higashi-Omiya Shiraoka Kuki Kasukabe Kawama Nanakodai Yagisaki Obukuro Koshigaya Atago Noda-Shi Umesato Unga Edogawadai Hatsuisi Toyoshiki Fukiage Kita-Konosu JR Takasaki Line Okegawa Ageo Ichinowari Nanasato Iwatsuki Higashi-Iwatsuki Toyoharu Takesato Sengendai Kita-Koshigaya Minami-Koshigaya Owada Tobu-Noda Line Kita-Kogane Kashiwa Abiko Kumagaya Gyoda Konosu Kitamoto Kitaageo Tobu Nota Line Toro Omiya-Koen Tennodai Miyahara Higashi-Urawa Higashi-Kawaguchi JR Musashino Line Misato Minami-Nagareyama Urawa Shin-Koshigaya Minami- Kita- Saitama- JR Tohoku HonsenKita-Omiya Warabi Nishi-Kawaguchi Kawaguchi Kashiwa Kashiwa Hon-Kawagoe Matsudo Shin- Gamo Takenozuka Yoshikawa Shin-Misato Shintoshin Nishi-Arai Umejima Mabashi Minoridai Gotanno Yono Kita-Urawa Minami-Urawa Kita-Akabane Akabane Shinden Yatsuka Shin- Musashiranzan Higashi-Jujo Kita-Matsudo Shinrin-koenHigashi-MatsuyamaTakasaka Omiya Kashiwa Toride Yono Minami- Honmachi Yono- Matsubara-Danchi Shin-Itabashi Minami-FuruyaJR Kawagoe Line Musashishi-Urawa Kita-Toda Toda Toda-Koen Ukima-Funato Kosuge JR Saikyo Line Shimo Matsudo Kita-Sakado Kita- Shimura- Akabane-Iwabuchi Soka Masuo Ogawamachi Naka-Urawa Takashimadaira Shiden Matsudo- Yono Nishi-Takashimadaira Hasune Sanchome Itabashi-Honcho Oji Kita-senju Kami- Myogaku Sashiogi Nisshin Nishi-Urawa Daishimae Tobu Isesaki Line Kita-Ayase Kanamachi Hongo Shimura- Jujo Oji-Kamiya Oku Sakasai Yabashira Kawagoe Shingashi Fujimino Tsuruse -

Bonus 2: International Schools the One & Only Guide You Need Tokyo Expat Job Search Guide
Bonus 2: International Schools The One & Only Guide You Need Tokyo Expat Job Search Guide Bonus 2: International Schools RAINER MORITA BONUS 2 INTERNATIONAL SCHOOLS Below you find a list of international schools for your children: American School in Japan 1-1-1 Nomizu, Chofu City, 1-1-2 Tokyo 1-1-3 Tel: (0422) 34-5300 www.asij.ac.jp Map 2 BONUS 2: INTERNATIONAL SCHOOLS Aoba-Japan International School Hikarigaoka Campus 7-5-1 Hikarigaoka, Nerima-ku, Tokyo Tel: (03) 6904-3127 www.aobajapan.jp Map 3 TOKYO EXPAT JOB SEARCH GUIDE Aoba-Japan International School Shoto Campus 2-2-1 Shoto, Shibuya-ku, Tokyo Tel: (03) 5738-6556 www.aobajapan.jp Map 4 BONUS 2: INTERNATIONAL SCHOOLS British School in Tokyo 1-21-18 Shibuya, Shibuya-ku, Tokyo Tel: (03) 5467-4321 www.bst.ac.jp Map 5 TOKYO EXPAT JOB SEARCH GUIDE Canadian International School 5-8-20 Kita Shinagawa, Shinagawa-ku, Tokyo Tel: (03) 5793-3839 www.cisjapan.net Map 6 BONUS 2: INTERNATIONAL SCHOOLS Christian Academy in Japan 1-2-14 Shinkawacho, Higashi Kurume City, Tokyo Tel: (0424) 71-0022 www.caj.or.jp Map 7 TOKYO EXPAT JOB SEARCH GUIDE Columbia International School 153 Matsugo, Tokorozawa City, Saitama Tel: (04) 2946-1911 www.columbia-ca.co.jp Map 8 BONUS 2: INTERNATIONAL SCHOOLS Deutsche School Tokyo Yokohama 2-4-1 Chigasaki Minami, Tsuzuki-ku, Yokohama Tel: (045) 941-4841 www.dsty.jp Map 9 TOKYO EXPAT JOB SEARCH GUIDE Eton House International Pre-School Tokyo 9-2-16 Akasaka, Minato-ku, Tokyo Tel: (03) 6804-3322 www.etonhouse.co.jp Map 10 BONUS 2: INTERNATIONAL SCHOOLS Global Kids Academy -

University of Tokyo Yasuyuki Matsuda, Assoc
Introduction to the University of Tokyo Yasuyuki Matsuda, Assoc. Prof. (Deputy Director, International Admission Office, UTokyo) What is your dream university? University is where… you gain professional knowledge and skills you explore the academic world with leading researchers you make life-long friends you immerse in new social/cultural environment with safe and comfortable living environment affordable living expenses UTokyo can offer all of them! Introduction to the University of Tokyo (18th November 2014) UTokyo can be your dream university Why Japan? Why Tokyo? Why UTokyo? What PEAK can offer to you? Introduction to the University of Tokyo (18th November 2014) Introduction of the city of Tokyo Introduction to the University of Tokyo (18th November 2014) Tokyo is… one of the largest cities in the world, with diverse cultural activities. Name of the city Population (million) Tokyo* 37.83 Delhi 24.95 Shanghai 22.99 Mexico City 20.84 Sao Paulo 20.83 Mumbai 20.74 Osaka 20.12 Beijing 19.52 New York 18.59 Sources: the U.S. Census Bureau and Times Atlas of the World Introduction to the University of Tokyo (18th November 2014) Tokyo is… one of the global financial hubs as well. Name of the stock Market Capitalization exchange (USD bn) New York Stock Exchange 18,779 NASDAQ 6,683 Tokyo Stock Exchange 4,485 Euronext 3,504 London Stock Exchange 3,396 Hong Kong Stock Exchange 3,146 Shanghai Stock Exchange 2,869 Toronto Stock Exchange 2,204 Sources: the world federation of exchanges monthly report Sep. 2014 Introduction to the University -
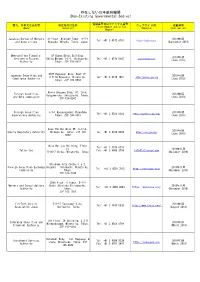
Non-Existing Governmental Bodies)
存在しない日本政府機関 (Non-Existing Governmental Bodies) 電話番号又はファックス番号 商号、名称又は氏名等 所在地又は住所 ウェブサイトURL 掲載時期 (Phone Number and/or Fax (Name) (Location) (Website) (Publication) Number) Japanese Bureau of Mergers 31 floor, Midtown Tower, 9-7-1 2019年9月 Tel: +81 3 4572 0701 https://japbma.org/ and Acquisitions Akasaka, Minato, Tokyo, Japan (September 2019) Metropolitan Financial 8F Humax Ebisu Building, 2019年6月 Services & Futures Ebisu Minami 1-1-1, Shibuya-ku, Tel: +81 3 4579 5647 www.mfinsfa.com (June 2019) Authority Tokyo, ZIP 150-0022 STEP Roppongi Bldg. West 1F, Japanese Securities and 2019年6月 6-8-10 Roppongi, Minato-ku, Tel: +81 3 4510 7897 http://jaseca-gov.org Compliance Authority (June 2019) Tokyo, ZIP 106-0032 World Udagawa Bldg. 6F, 36-6, Foreign Securities 2019年6月 Udagawa-cho, Shibuya-ku, Tokyo, and Bond commission (June 2019) ZIP 150-0042 Foreign Securities 3-7-1 Kasumigaseki,Chiyodaku 2019年6月 Tel: +81 3 4520 8922 https://www.fssa-gov.org/ Supervisory Authority Tokyo, ZIP 100-0013 (June 2019) Axes 7th Building 6F, 3-17-4, 2019年6月 Equity Regulatory Authority Shibuya-ku, Tokyo, ZIP 150- Tel: +81 3 4520 8934 https://era-gov.org/ (June 2019) 0002 Mita Belljyu Building, Floor Tel: +81 3 4579 0731 24, 2018年11月 Tatler Cox Fax: +81 3 6800 2769 [email protected] 5-36-7 Shiba, Minato-ku, Tokyo (November 2018) Shiodome City Center 1-5-2, Foreign Securities Exchange Higashi - Shinbashi, Minato-ku, 2018年11月 Tel: +81 3 4510 7815 http://fsec-gov.org/ Commission Tokyo, (November 2018) ZIP 105-7140 29th floor, C tower, 3-7-1 Mergers and Consolidations Nishi -

Comforia Residential REIT, Inc 21-1 Dogenzaka 1-Chome, Shibuya-Ku,Tokyo Takehiro Izawa Executive Director (Code: 3282)
March 12, 2021 For Immediate Release Real Estate Investment Trust Securities Issuer: Comforia Residential REIT, Inc 21-1 Dogenzaka 1-chome, Shibuya-ku,Tokyo Takehiro Izawa Executive Director (Code: 3282) Asset Management Company: TLC REIT Management Inc. Hiroyuki Tohmata President & CEO Inquiries: Kentaro Yoshikawa General Manager of Strategy Department Comforia Management Division (TEL: +81-3-6455-3388) Notice Concerning Acquisition and Sale of Investment Assets Comforia Residential REIT, Inc (“CRR”) announces that TLC REIT Management Inc. (“TRM”), to which CRR entrusts management of its assets decided today for CRR to acquire the investment asset as shown below 1 (1), and sell the investment asset as shown below 1 (2). (hereafter referred to as the “Acquisition” and the “Sale” respectively and the “Transactions” collectively). 1. Summary of the Transactions (1) Summary of the Acquisition Acquisition Price No. Type of Asset Property Name (thousand yen) (Note1) Beneficial Interest in 1 COMFORIA OMIYA(Note2) 4,420,300 Real Estate Trust Total 4,420,300 (Note1) “Acquisition Price” denotes the amount exclusive of the various expenses required in the acquisition of the concerned asset, etc. (brokerage commission, taxes and public dues, etc.) (the amount of real estate or beneficial interest in real estate trust specified in the Agreement on Purchase and Sale). (Note2) Although the current property name is “SAION OMIYA”, CRR plans to change the name to “COMFORIA OMIYA” about one month after the acquisition. The current property name will be -

Register of Medical Institutions Issuing COVID-19 Testing
Register of Medical Institutions Issuing COVID-19 Testing Certificates as of September 20th, 2021 【About Antigen test kit (qualitative antigen test)】 ・In Japan, PCR Test including LAMP Method and Quantitative Antigen Test are permitted for asymptomatic patient as appropriate test method, but Antigen test kit (qualitative antigen test) are not permitted. ・At the request of the destination, Antigen test kit (qualitative antigen test) is used for asymptomatic patient, and if the test result is positive, PCR Test or other appropriate test method may be performed based on doctor's judgement. ※Permitted test method in Japan are highlighted in light blue at the table below. ※Reference: Guidelines for COVID-19 Pathogen Test Basic Information of Medical Institution Inspection Information Contact Address Testing Methods for Issuing a Certificate Information TeCOT PCR Testing PCR Testing Antigen Testing Antigen Testing No Reservation LAMP Method Other Methods Real-Time Method Non-Real-Time Method Simple Kit Quantitative Availability Medical Institution Name Phone Prefecture Municipality Street Address Nasopharynx Saliva Nasopharynx Saliva Nasopharynx Saliva Nasopharynx Saliva Nasopharynx Saliva Nasopharynx Saliva Number Min. Req. Min. Req. Min. Req. Min. Req. Min. Req. Min. Req. Min. Req. Min. Req. Min. Req. Min. Req. Min. Req. Min. Req. Availability Availability Availability Availability Availability Availability Availability Availability Availability Availability Availability Availability Time Time Time Time Time Time Time Time Time Time Time Time -

Similarity of Muscle Synergies Extracted from the Lower Limb
Gait & Posture 59 (2018) 134–139 Contents lists available at ScienceDirect Gait & Posture journal homepage: www.elsevier.com/locate/gaitpost Full length article Similarity of muscle synergies extracted from the lower limb including the MARK deep muscles between level and uphill treadmill walking ⁎ Akira Saitoa,b, , Aya Tomitac, Ryosuke Andob,d, Kohei Watanabee, Hiroshi Akimac,d a Graduate School of Arts and Sciences, The University of Tokyo, Komaba, Meguro-ku, Tokyo, Japan b Japan Society for the Promotion of Science, Kojimachi, Chiyoda-ku, Tokyo, Japan c Graduate School of Education and Human Development, Nagoya University, Furo-cho, Chikusa-ku, Nagoya, Aichi, Japan d Research Center of Health, Physical Fitness & Sports, Nagoya University, Furo-cho, Chikusa-ku, Nagoya, Aichi, Japan e School of International Liberal Studies, Chukyo University, Yagotohonmachi, Showa-ku, Nagoya, Aichi, Japan ARTICLE INFO ABSTRACT Keywords: This study aimed to examine muscle synergies involving the deeper muscles of the lower limb during level and Surface electromyography uphill treadmill walking. Seven men and five women walked on a treadmill at three speeds (60, 80, and 100 m/ Non-negative matrix factorization min) and two grades (level and 10% grade). Surface electromyographic (EMG) signals were recorded from 10 Gait analysis muscles of the lower limb, including vastus intermedius, adductor magnus, and adductor longus. Muscle sy- Vastus intermedius nergies were extracted applying non-negative matrix factorization, and the relative co-activation across muscles Hip adductors and the temporal information of synergy recruitment were identified by the muscle synergy vector and synergy Ultrasonography activation coefficient, respectively. Correlation coefficients between a pair of synergy vectors during level and uphill walking were analyzed as a similarity index, with the similarity criterion at r = 0.76. -

Relationship Between Physical Activity and Intramyocellular Lipid Content Is Different Between Young and Older Adults
European Journal of Applied Physiology (2019) 119:113–122 https://doi.org/10.1007/s00421-018-4005-x ORIGINAL ARTICLE Relationship between physical activity and intramyocellular lipid content is different between young and older adults Maya Hioki1 · Nana Kanehira2 · Teruhiko Koike3 · Akira Saito4,5 · Hideyuki Takahashi6 · Kiyoshi Shimaoka7 · Hisataka Sakakibara1 · Yoshiharu Oshida3 · Hiroshi Akima3 Received: 26 March 2018 / Accepted: 1 October 2018 / Published online: 10 October 2018 © Springer-Verlag GmbH Germany, part of Springer Nature 2018 Abstract Purpose Intramyocellular lipid (IMCL) is influenced by physical exercise; however, whether the habitual level of physical activity affects resting IMCL content remains unclear. The purpose of this study was to determine the relationship between physical activity levels and resting IMCL content in young and older adults. Methods In total, 15 nonobese young adults (21.0 ± 0.0 years) and 15 older adults (70.7 ± 3.8 years) were recruited. Time spent performing physical activities for 10 days was assessed using a three-dimensional ambulatory accelerometer, and inten- sity was categorized as light [< 3.0 metabolic equivalents (METs)], moderate (3.0–6.0 METs), or vigorous (> 6.0 METs). Physical activity level was calculated as the product of METs and time spent performing physical activities (MET h) at each intensity level. The IMCL content in the vastus lateralis was determined using 1H-magnetic resonance spectroscopy after overnight fasting. Results No significant differences in IMCL content were observed between young and older adults. Vigorous intensity physi- cal activity (time and MET h) was significantly lower in older than young adults (p < 0.01); this difference was not observed for light and moderate intensity physical activity. -

Mejirodai International Village Tokyo Metro Kishimojin Yurakucho Line
Otsuka Otsuka ekimae Ikebukuro sta. Sunshine 60 Mukohara Otsuka Tokyo Metro Marunouchi Line Toden Arakawa Line Higashi Ikebukuro Shin Otsuka The University of Tokyo Tokyo Metro Higashi Ikebukuro Yonchome Fukutoshin Line Toden-zoshigaya Mejirodai International Village Tokyo Metro Kishimojin Yurakucho Line Tokyo Metropolitan Expressway Kishimojinmae Route 5 Ikebukuro Line September 2019 Zoshigaya UTokyo, Gokokuji Mejirodai International Village ● 5 min. walk from Gokokuji Station (Tokyo Metro Yurakucho Line) ●16 min. walk from Myogadani Station (Tokyo Metro Marunouchi Line) Myogadani Gakushuinshita ① Convenience store (Facility in the hall) ③ Maruetsu (Supermarket, 7 min walk) ⑤ Kodansha (5 min walk) ⑦ Mejirodai Sports Park (6 min walk) ② Family restaurant (6 min walk) ④ Otsuka Police Station (3 min walk) ⑥ Gokokuji Temple (8 min walk) ⑧ Tokyo Metropolitan Ohtsuka Hospital (18 min walk) Property Description Address: 3-28-6 Mejirodai, Bunkyo-ku, Tokyo Structure: Pre-stressed concrete and steel frame structure + Reinforced concrete structure Access: 5 minute walk from Gokokuji Station (Tokyo Metro Yurakucho line) Auxiliary facilities: Restaurant, convenience store and joint industry-university facility Number of rooms: 857 rooms in total Management system: 24 hours manned management 704 rooms in shared type dwelling units, 153 rooms in independent type dwelling units Bicycle parking area: Available Eligibility: Japanese Students, International Students, International Curfew: None Researchers enrolled at UTokyo Application period: Refer to the attached sheet. Accommodation Fees: Refer to the attached sheet. Period of moving-in: Scheduled on September 2019 Access to each campus Tokyo Metro Tobu Tojo Line Tokyo Metro Nanboku Line Kashiwa Campus Yurakucho Line ■ Komaba Campus Fukutoshin Line Kashiwanoha (Komabatodaimae sta. Keio Inokashira Line) Komagome Campus approx. -

Financial Services Agency Kanto Local Finance Bureau(FIBO) No.16 AXA INVESTMENT MANAGERS JAPAN LTD
Financial Instruments Business Operators As of August 31, 2021 Type I Type II Investment Investment Securities- Financial Financial Advisory and Jurisdiction Registration numbers Name JCN Address Telephone Management Related Instruments Instruments Agency Business Business Business Business Business Financial Services Agency Kanto Local Finance Bureau(FIBO) No.16 AXA INVESTMENT MANAGERS JAPAN LTD. 4010401061149 1-17-3, Shirokane, Minato-ku, Tokyo 03-5447-3100 ○ ○ ○ ○ ○ Kanto Local Finance Bureau(FIBO) No.18 Ichigo Estate Co., Ltd. 1010001125620 1-1-1,Uchisaiwai-cho,Chiyoda-ku,Tokyo 03-3502-5625 ○ ○ ○ Kanto Local Finance Bureau(FIBO) No.44 SBI Securities CO., LTD. 3010401049814 1-6-1, Roppongi, Minato-ku, Tokyo 03-5562-7210 ○ ○ ○ ○ Kanto Local Finance Bureau(FIBO) No.45 Japannext Co.,Ltd. 2010001139289 3-1-1, Roppongi, Minato-ku, Tokyo 03-4577-4040 ○ ○ Kanto Local Finance Bureau(FIBO) No.51 Yensai.com Co., Ltd. 8010001082954 1-16-1, Kanda-Nishikicho, Chiyoda-ku, Tokyo 03-3518-8011 ○ ○ Kanto Local Finance Bureau(FIBO) No.52 Okasan Online Securities Co.,Ltd 2010001097479 3-9-7, Ginza, Chuo-ku, Tokyo 03-3547-0100 ○ ○ ○ ○ Kanto Local Finance Bureau(FIBO) No.53 OKASAN SECURITIES CO.,LTD. 5010001082560 1-17-6,Nihonbashi,Chuo-ku,Tokyo 03-3272-2211 ○ ○ ○ ○ Kanto Local Finance Bureau(FIBO) No.57 Janus Henderson Investors (Japan) Limited 3010001012362 1-6-5,Marunouchi, Chiyoda-ku, Tokyo 03-5219-8000 ○ ○ ○ Kanto Local Finance Bureau(FIBO) No.61 au Kabucom Securities Co., Ltd. 5010001066670 1-3-2, Otemachi, Chiyoda-ku, Tokyo 03-4221-3551 ○ ○ ○ ○ Kanto Local Finance Bureau(FIBO) No.66 Credit Suisse Securities (Japan) Limited 2010401059260 1-6-1,Roppongi Minato-ku,Tokyo 03-4550-9000 ○ ○ ○ ○ ○ Kanto Local Finance Bureau(FIBO) No.69 Goldman Sachs Japan Co.,Ltd.