Epidemiological Study of Ticks and Their Distribution in Decha Woreda of Kafa Zone, SNNPRS
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Districts of Ethiopia
Region District or Woredas Zone Remarks Afar Region Argobba Special Woreda -- Independent district/woredas Afar Region Afambo Zone 1 (Awsi Rasu) Afar Region Asayita Zone 1 (Awsi Rasu) Afar Region Chifra Zone 1 (Awsi Rasu) Afar Region Dubti Zone 1 (Awsi Rasu) Afar Region Elidar Zone 1 (Awsi Rasu) Afar Region Kori Zone 1 (Awsi Rasu) Afar Region Mille Zone 1 (Awsi Rasu) Afar Region Abala Zone 2 (Kilbet Rasu) Afar Region Afdera Zone 2 (Kilbet Rasu) Afar Region Berhale Zone 2 (Kilbet Rasu) Afar Region Dallol Zone 2 (Kilbet Rasu) Afar Region Erebti Zone 2 (Kilbet Rasu) Afar Region Koneba Zone 2 (Kilbet Rasu) Afar Region Megale Zone 2 (Kilbet Rasu) Afar Region Amibara Zone 3 (Gabi Rasu) Afar Region Awash Fentale Zone 3 (Gabi Rasu) Afar Region Bure Mudaytu Zone 3 (Gabi Rasu) Afar Region Dulecha Zone 3 (Gabi Rasu) Afar Region Gewane Zone 3 (Gabi Rasu) Afar Region Aura Zone 4 (Fantena Rasu) Afar Region Ewa Zone 4 (Fantena Rasu) Afar Region Gulina Zone 4 (Fantena Rasu) Afar Region Teru Zone 4 (Fantena Rasu) Afar Region Yalo Zone 4 (Fantena Rasu) Afar Region Dalifage (formerly known as Artuma) Zone 5 (Hari Rasu) Afar Region Dewe Zone 5 (Hari Rasu) Afar Region Hadele Ele (formerly known as Fursi) Zone 5 (Hari Rasu) Afar Region Simurobi Gele'alo Zone 5 (Hari Rasu) Afar Region Telalak Zone 5 (Hari Rasu) Amhara Region Achefer -- Defunct district/woredas Amhara Region Angolalla Terana Asagirt -- Defunct district/woredas Amhara Region Artuma Fursina Jile -- Defunct district/woredas Amhara Region Banja -- Defunct district/woredas Amhara Region Belessa -- -

Fissha Asmare.Pdf
SCHOOL OF GRADUATE STUDIES SCHOOL OF ECONOMICS THE EFFECT OF CLIMATE CHANGE ADAPTATION STRATEGY ON FARM HOUSEHOLD’S WELFARE IN NILE BASIN OF ETHIOPIA: IS THERE SYNERGY OR TRADEOFF? BY FISSHA ASMARE MARYE ADDIS ABABA, ETHIOPIA JUNE, 2016 ADDIS ABABA UNIVERSITY SCHOOL OF GRADUATE STUDIES SCHOOL OF ECONOMICS The Effect of Climate Change Adaptation Strategy on Farm Household’s Welfare in Nile Basin of Ethiopia: Is There Synergy or Tradeoff? A Thesis Submitted To the School Of Graduate Studies of Addis Ababa University in Partial Fulfillment of the Requirements for the Degree Of Master of Science in Economics (Resource and Environmental Economics Stream) By Fissha Asmare Marye Addis Ababa, Ethiopia June, 2016 Addis Ababa University School of Graduate Studies This is to certify that the thesis prepared by Fissha Asmare, entitled: the effect of climate change adaptation strategy on farm household’s welfare in Nile basin of Ethiopia: is there synergy or trade off?; and submitted in partial fulfillment of the requirement for the degree of Masters of Science in Economics (Resource and Environmental Economics) complies with the regulations of the university and meets the accepted standards with respect to originality and quality. Signed by the examining committee: Chairman: Kebede Bekele signature_______________ Date: ____________________________ External Examiner: Abebe Damte (PhD) signature_____________ Date: ____________________________ Internal Examiner: Adane Tuffa (PhD) signature______________ Date: ____________________________ Advisor: Hailemariam Teklewold (PhD) signature______________ Date: ____________________________ Graduate programs coordinator, Department of Economics Kebede Bekele: signature ____ Date: ____________________________ i ACKNOWLEDGEMENTS First I would like to thank God Almighty; it is not exaggeration to say that without insisting the Almighty God would not have been in a place to complete successfully this thesis paper. -

World Bank Document
Sample Procurement Plan (Text in italic font is meant for instruction to staff and should be deleted in the final version of the PP) Public Disclosure Authorized (This is only a sample with the minimum content that is required to be included in the PAD. The detailed procurement plan is still mandatory for disclosure on the Bank’s website in accordance with the guidelines. The initial procurement plan will cover the first 18 months of the project and then updated annually or earlier as necessary). I. General 1. Bank’s approval Date of the procurement Plan: Updated Procurement Plan, M 2. Date of General Procurement Notice: Dec 24, 2006 Public Disclosure Authorized 3. Period covered by this procurement plan: The procurement period of project covered from year June 2010 to December 2012 II. Goods and Works and non-consulting services. 1. Prior Review Threshold: Procurement Decisions subject to Prior Review by the Bank as stated in Appendix 1 to the Guidelines for Procurement: [Thresholds for applicable procurement methods (not limited to the list below) will be determined by the Procurement Specialist /Procurement Accredited Staff based on the assessment of the implementing agency’s capacity.] Public Disclosure Authorized Procurement Method Prior Review Comments Threshold US$ 1. ICB and LIB (Goods) Above US$ 500,000 All 2. NCB (Goods) Above US$ 100,000 First contract 3. ICB (Works) Above US$ 15 million All 4. NCB (Works) Above US$ 5 million All 5. (Non-Consultant Services) Below US$ 100,000 First contract [Add other methods if necessary] 2. Prequalification. Bidders for _Not applicable_ shall be prequalified in accordance with the provisions of paragraphs 2.9 and 2.10 of the Public Disclosure Authorized Guidelines. -

Resettlement and Sustainable Livelihoods in Ethiopia: a Comparative Analysis of Amhara And
RESETTLEMENT AND SUSTAINABLE LIVELIHOODS IN ETHIOPIA: A COMPARATIVE ANALYSIS OF AMHARA AND SOUTHERN REGIONS BY KASSA TESHAGER ALEMU Submitted in accordance with the requirements For the degree of DOCTOR OF LITERATURE AND PHILOSOPHY In the subject DEVELOPMENT STUDIES At the UNIVERSITY OF SOUTH AFRICA SUPERVISOR: DR SEVENIA VICTOR PETER MADZIAKAPITA February 2015 i DECLARATION I, KASSA TESHAGER ALEMU, do hereby declare that this doctoral thesis titled “RESETTLEMENT AND SUSTAINABLE LIVELIHOODS IN ETHIOPIA: A Comparative Analysis of Amhara and Southern Regions” is my own work and that all the sources that I have used or quoted have been indicated and acknowledged by means of complete references. In addition, I also declare that this work has not been submitted elsewhere for a similar or any other educational or non-educational award. Candidate: Kassa Teshager Alemu Signature: __________________ Date: __________________ ii ACKNOWLEDGEMENT I express my gratitude to the University of South Africa (UNISA), Department of Development Studies, for giving me the chance to do my PhD in Development Studies. I appreciate the UNISA- Ethiopia Branch staff members and UNISA-SANTRUST PhD proposal development programme coordinators and trainers for the hard work and support they provided in this study. I sincerely thank the School of Graduate Studies, Ethiopian Civil Service University, for the funding and study leave provided. I thank the Institute of Public Management and Development Studies (IPMDS), Department of Development Management, for providing multi-dimensional support and giving me a pleasant working environment. I am also grateful to the Nordic Africa Institute (NAI) in Sweden for providing me the African Guest Researchers Scholarship of 2014. -

The Role of Saving and Credit Cooperatives in Improving Rural Micro Financing: the Case of Bench Maji, Kaffa, Shaka Zones
World Journal of Business and Management ISSN 2377-4622 2018, Vol. 4, No. 2 The Role of Saving and Credit Cooperatives in Improving Rural Micro Financing: The Case of Bench Maji, Kaffa, Shaka Zones Mr. Tesfaye Megiso Begajo Department of Cooperatives, College of Business and Economics, Mizan-Tepi University PO Box 260, Mizan Teferi, Ethiopia Tel: 251-946-511-347 E-mail: [email protected] Received: November 2, 2018 Accepted: December 6, 2018 Published: December 10, 2018 doi:10.5296/wjbm.v4i2.13849 URL: https://doi.org/10.5296/wjbm.v4i2.13849 Abstract Microfinance is the provision of microloans to poor entrepreneurs and small businesses lacking access to banking and related services. Saving and Credit Cooperative are the main source of finance for people who have low income level. In this study the role of saving and credit cooperatives in improving rural micro financing in Bench Maji, Kaffa, and Shaka Zones was examined. Methodologically descriptive survey research was applied. Ideological preparedness of community on saving, tool to improve members saving culture, members’ actual deposit, and loan facilities performed by cooperatives were the focuses in this study. The findings show that there is a contribution in changing the saving culture of the members, monthly deposit, and loan disbursement capacity. The challenges are shortage of finance for loan, irregularity in saving, weak collection of loan receivables, no collaboration with other financial institutions, limitation in awareness creation, and limited support of government to lower administrative level. Therefore, to fulfil the members’ financial need cooperatives have to increase the number of members, the Government should establish strong education, training, and information unit at Zone and Woreda level, without abiding cooperative rules should arrange capital injection and collaboration with Micro financial Institutions, private and public banks. -

Demography and Health
SNNPR Southern Nations Nationalities and Peoples Demography and Health Aynalem Adugna, July 2014 www.EthioDemographyAndHealth.Org 2 SNNPR is one of the largest regions in Ethiopia, accounting for more than 10 percent of the country’s land area [1]. The mid-2008 population is estimated at nearly 16,000,000; almost a fifth of the country’s population. With less than one in tenth of its population (8.9%) living in urban areas in 2008 the region is overwhelmingly rural. "The region is divided into 13 administrative zones, 133 Woredas and 3512 Kebeles, and its capital is Awassa." [1] "The SNNPR is an extremely ethnically diverse region of Ethiopia, inhabited by more than 80 ethnic groups, of which over 45 (or 56 percent) are indigenous to the region (CSA 1996). These ethnic groups are distinguished by different languages, cultures, and socioeconomic organizations. Although none of the indigenous ethnic groups dominates the ethnic makeup of the national population, there is a considerable ethnic imbalance within the region. The largest ethnic groups in the SNNPR are the Sidama (17.6 percent), Wolayta (11.7 percent), Gurage (8.8 percent), Hadiya (8.4 percent), Selite (7.1 percent), Gamo (6.7 percent), Keffa (5.3 percent), Gedeo (4.4 percent), and Kembata (4.3 percent) …. While the Sidama are the largest ethnic group in the region, each ethnic group is numerically dominant in its respective administrative zone, and there are large minority ethnic groups in each zone. The languages spoken in the SNNPR can be classified into four linguistic families: Cushitic, Nilotic, Omotic, and Semitic. -
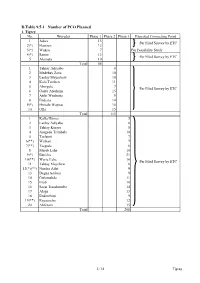
D.Table 9.5-1 Number of PCO Planned 1
D.Table 9.5-1 Number of PCO Planned 1. Tigrey No. Woredas Phase 1 Phase 2 Phase 3 Expected Connecting Point 1 Adwa 13 Per Filed Survey by ETC 2(*) Hawzen 12 3(*) Wukro 7 Per Feasibility Study 4(*) Samre 13 Per Filed Survey by ETC 5 Alamata 10 Total 55 1 Tahtay Adiyabo 8 2 Medebay Zana 10 3 Laelay Mayechew 10 4 Kola Temben 11 5 Abergele 7 Per Filed Survey by ETC 6 Ganta Afeshum 15 7 Atsbi Wenberta 9 8 Enderta 14 9(*) Hintalo Wajirat 16 10 Ofla 15 Total 115 1 Kafta Humer 5 2 Laelay Adiyabo 8 3 Tahtay Koraro 8 4 Asegede Tsimbela 10 5 Tselemti 7 6(**) Welkait 7 7(**) Tsegede 6 8 Mereb Lehe 10 9(*) Enticho 21 10(**) Werie Lehe 16 Per Filed Survey by ETC 11 Tahtay Maychew 8 12(*)(**) Naeder Adet 9 13 Degua temben 9 14 Gulomahda 11 15 Erob 10 16 Saesi Tsaedaemba 14 17 Alage 13 18 Endmehoni 9 19(**) Rayaazebo 12 20 Ahferom 15 Total 208 1/14 Tigrey D.Table 9.5-1 Number of PCO Planned 2. Affar No. Woredas Phase 1 Phase 2 Phase 3 Expected Connecting Point 1 Ayisaita 3 2 Dubti 5 Per Filed Survey by ETC 3 Chifra 2 Total 10 1(*) Mile 1 2(*) Elidar 1 3 Koneba 4 4 Berahle 4 Per Filed Survey by ETC 5 Amibara 5 6 Gewane 1 7 Ewa 1 8 Dewele 1 Total 18 1 Ere Bti 1 2 Abala 2 3 Megale 1 4 Dalul 4 5 Afdera 1 6 Awash Fentale 3 7 Dulecha 1 8 Bure Mudaytu 1 Per Filed Survey by ETC 9 Arboba Special Woreda 1 10 Aura 1 11 Teru 1 12 Yalo 1 13 Gulina 1 14 Telalak 1 15 Simurobi 1 Total 21 2/14 Affar D.Table 9.5-1 Number of PCO Planned 3. -

Ecx Coffee Contracts
ECX COFFEE CONTRACTS 1. CONTRACT CTASSIFICATIONS AND DELIVERY CENTRES 1.1. EXPORT - SPECIALTY - WASHED Origin (Woreda or Zone) Delivery Coffee Contract Symbol Grades Centre YIRGACHEFE A- Yirqachefe WYCA Ql, Q2 Dilla WENAGO A- Wenaqo Q1,Q2 Dilla KOCHERE A- Kochere WKCA Q1,Q2 Dilla GELENA ABAYA A- Gelena/Abaya WGAA Q1,Q2 Dilla YIRGACHEFE B** Yirqachefe WYCB Q1,Q2 Dilla WENAGO B-- Wenago WWNB Q1,Q2 Dilla KOCHERE B-- Kochere WKCB Q1,Q2 Dilla CELENA ABAYA B* Gelena/Abaya WGAB Q1,Q2 Dilla GUJI Oddo Shakiso, Addola Redi, Uraga, Kercha, WGJ Q1,Q2 HawasSa Bule Hora Borena( except Gelena/Abaya), Benssa, Chire, Bona SIDAMA A WSDA Q1,Q2 Hawassa zu ria, Arroressa, Arbiqona Aleta Wendo, Dale, Chuko, Dara, Shebedino, SIDAMA B WSDB Q1,Q2 Hawassa Wensho, Loko Abava, Amaro, Dilla zuria SIDAMA C Kembata &Timbaro, Wollaita WSDC o1,Q2 Soddo SIDAMA D West Arsi (Nansebo), Arsi (Chole), Bale WSDD Hawassa Soddo SIDAMA E S.Ari, N.Ari, Melo, Denba gofa, Geze gofa, WSDE Q1,Q2 Arbamlnch zuria, Basketo, Derashe, Konso, Konta. Gena bosa, Esera Limmu Seka, Limmu Kossa, Manna, Gomma, WLMA J rmma UMMU A Gummav, Seka Chekoressa, Kersa, Shebe,Gera Q1,Q2 Bedelle, Noppa, Chona, YaYo, AIle, didu, Bedelle LIMMU B WLMB Q1,Q2 Dedessa, KAFFA Glmbo, Gewata, Chena, Tilo, Bita, Cheta, WKF Q1,Q2 Bonga Gesha GODERE Mezenge(Godere) WGD Q1,Q2 Bonqa YEKI Yeki WYK Q1,Q2 Bonqa ANDERACHA Anderacha WAN Q1,Q2 Bonga BENCH MAJI Sheko, S.Bench, N.Bench, Gura ferda, Bero WBM Q1, Q2 Bonga BEBEKA Bebeka WBB QT, Q2 Bonqa KELEM WELEGA Kelem Wolleqa Q1, Q2 Gim bi EAST WELLEGA East Wolleqa WEW Gimbi -

Ethiopia Administrative Map As of 2013
(as of 27 March 2013) ETHIOPIA:Administrative Map R E Legend E R I T R E A North D Western \( Erob \ Tahtay Laelay National Capital Mereb Ahferom Gulomekeda Adiyabo Adiyabo Leke Central Ganta S Dalul P Afeshum Saesie Tahtay Laelay Adwa E P Tahtay Tsaedaemba Regional Capital Kafta Maychew Maychew Koraro Humera Asgede Werei Eastern A Leke Hawzen Tsimbila Medebay Koneba Zana Kelete Berahle Western Atsbi International Boundary Welkait Awelallo Naeder Tigray Wenberta Tselemti Adet Kola Degua Tsegede Temben Mekele Temben P Zone 2 Undetermined Boundary Addi Tselemt Tanqua Afdera Abergele Enderta Arekay Ab Ala Tsegede Beyeda Mirab Armacho Debark Hintalo Abergele Saharti Erebti Regional Boundary Wejirat Tach Samre Megale Bidu Armacho Dabat Janamora Alaje Lay Sahla Zonal Boundary Armacho Wegera Southern Ziquala Metema Sekota Endamehoni Raya S U D A N North Wag Azebo Chilga Yalo Amhara East Ofla Teru Woreda Boundary Gonder West Belesa Himra Kurri Gonder Dehana Dembia Belesa Zuria Gaz Alamata Zone 4 Quara Gibla Elidar Takusa I Libo Ebenat Gulina Lake Kemkem Bugna Kobo Awra Afar T Lake Tana Lasta Gidan (Ayna) Zone 1 0 50 100 200 km Alfa Ewa U Fogera North Farta Lay Semera ¹ Meket Guba Lafto Semen Gayint Wollo P O Dubti Jawi Achefer Bahir Dar East Tach Wadla Habru Chifra B G U L F O F A D E N Delanta Aysaita Creation date:27 Mar.2013 P Dera Esite Gayint I Debub Bahirdar Ambasel Dawunt Worebabu Map Doc Name:21_ADM_000_ETH_032713_A0 Achefer Zuria West Thehulederie J Dangura Simada Tenta Sources:CSA (2007 population census purpose) and Field Pawe Mecha -

Tourism Concept for Kafa Biosphere Reserve
Development of management and business plan for ecotourism in Kafa Biosphere Reserve, Bonga, Ethiopia In the frame of the NABU project: „Climate Protection and Preservation of Primary Forests – A Management Model using the Wild Coffee Forests in Ethiopia as an Example” Funded by the German Federal Ministry for the Environment, Nature Conservation and Nuclear Safety within the frame of the International Climate Initiative Final report by Sigrun Lange & Michael Jungmeier 21 October 2011 FINAL REPORT DEVELOPMENT OF ECOTOURISM IN KAFA BR Table of Contents List of acronyms ........................................................................................................................... 4 Preliminary notes......................................................................................................................... 5 1 Description of Kafa Biosphere Reserve .................................................................................. 6 2 Rationale for ecotourism development in Kafa Biosphere Reserve ......................................... 9 2.1 The hope for a bright future of tourism in Ethiopia .............................................................................. 9 2.2 Definition of (community-based) ecotourism ..................................................................................... 10 2.3 Tourism in southwest Ethiopia and Kafa Biosphere Reserve .............................................................. 11 2.4 SWOT analysis for ecotourism development in Kafa Biosphere Reserve........................................... -

(Ensete Ventricosum Welw. Cheesman) and Its Relation to Household Food and Livelihood Security in South-Western Ethiopia
2001-0©-12 uiversny and conservation of enset (Ensete ventricosum Welw. Cheesman) and its relation to household food and livelihood security in South-western Ethiopia Almaz Negash STELLINGEN belonging to the thesis Diversity and Conservation ofEnset (Ensete ventricosum Welw. Cheesman) and its Relation to Household Food and Livelihood Security in South-western Ethiopia Almaz Negash, 26 September 2001 1. The conservation of crop genetic diversity entails the actual genetic diversity together with the existing traditional knowledge, which nurtured the crop (this thesis). 2. The issues constituting and surrounding the problems of food security may appear complex and intractable, but they are manageable, and soluble. We need only the will at the level of policy, a more assertive and a more committed role at the level of the professional and a more free and a more decisive participation at the level of the peasantry (Mesfin Wolde-Mariam, 1999). 3. Increased production and consumption of indigenous crops such as enset will increase food supplies and may also broaden the food base at both household and national level (this thesis). 4. Conservation becomes more sensible and effective when it is linked to the food security and livelihood systems of the local communities (this thesis). 5. The Ethiopian proverb "A home without a woman is like a barn without cattle" indicates an awareness about the important role of women both in the house and on the farm. 6. Hardly anywhere are target groups taken as equal partners on the basis of respect for then- knowledge, technology, world views and capability (Arte, 1992). 7. The diversity contained in indigenous agriculture at crop, field and regional level offers greater yield stability than does the less diverse industrial agriculture (Cleveland, 1993). -

Addis Ababa University School of Graduate Studies
ADDIS ABABA UNIVERSITY SCHOOL OF GRADUATE STUDIES PERCEPTION ON SOCIO-ECONOMIC AND DEMOGRAPHIC CAUSES OF DEFORESTATION AND ITS CONSEQUENCES IN CHENA WOREDA: THE CASE OF KAFA ZONE, SNNPRS BY Tamirat Moges June, 2010 Addis Ababa, Ethiopia ADDIS ABABA UNIVERSITY SCHOOL OF GRADUATE STUDIES PERCEPTION ON SOCIO-ECONOMIC AND DEMOGRAPHIC CAUSES OF DEFORESTATION AND ITS CONSEQUENCES IN CHENA WOREDA: THE CASE OF KAFA ZONE, SNNPRS BY Tamirat Moges A THESIS SUBMITTED TO INSTITUTE OF POPULATION STUDIES IN PARTIAL FULFILMENT OF THE REQUIRENMENT FOR THE DEGREE OF MASTER OF SCINCE IN DEMOGRAPHY (POPULATION STUDIES) Acknowledgement I emphatically would like to thank the Almighty GOD for all His limitless support throughout my life. My strong gratitude also goes to my adviser Dr. Terefe Degefa for his unreserved assistance, constructive advise, guidance and fruitful suggestions by devoting his time and efforts for the better of the thesis. My heartfelt thank go to Chena Woreda Agricultural and Rural Development office, Health officer Ato Aklilu Mohamed who helped me by giving different valuable information and to the sample respondents, FGD members and my moderator and language translator Birhanu W/ Michael who cooperate with me throughout the process of information gathering. Finally I am grateful to all my families especially to Sinafikish Mamushet who encourages and supports highly and took much part in my educational career and life at all. TABLE OF CONTENT List of Contents Page A. Acknowledgement------------------------------------------------------------------i