BRIEF HISTORY of CHEMISTRY
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Mystical Practices of Alchemy
Mystical Practices of Alchemy The language of secret symbols has always hidden alchemy from the curiosity of the uninitiated. We still do not actually know alchemy’s true nature: for some it is the making of gold from other metals, for others – finding the elixir of immortality, and select groups throughout history have tried to accomplish complete transformation of the human body and soul. Royal art Alchemy is the mother of chemistry. It is in alchemical laboratories that for the first time scientists obtained sulfur, nitric and hydrochloric acid, saltpeter, lead, and many drugs. Medieval alchemists set very specific tasks. One of the European heads of alchemy, Rodger Bacon (13th century) wrote the following: “Alchemy is the science of how to prepare a compound or elixir, which, if added to base metals will transform them into sophisticated metals.” By transforming base metals into precious, alchemy challenges nature itself. Even though in Medieval Europe alchemy was practically illegal, many religious and secular people patronized it depending on hoping to get the “contemptible metal”. Not only did they patronize it, they actually practiced it themselves. Alchemy became a real “royal art”. Augustus II the Strong (1670-1733) whose claim of the Polish crown required significant resources transformed Dresden into the true capital of alchemy. For the purpose of filling the national treasury with gold, he brought the talented alchemist Johann Friedrich Böttger. To what extent did Böttger succeed in filling the treasury with gold, history remains silent. Alchemists in Europe were a lot, but the adepts – the ones who knew the secret of the Philosopher’s stone – were very few. -

Die Edelgeborne Jungfer Alchymia: the Final Stage of European Alchemy
50 Bull. Hist. Chem., VOLUME 25, Number 1 (2000) DIE EDELGEBORNE JUNGFER ALCHYMIA: THE FINAL STAGE OF EUROPEAN ALCHEMY Vladimír Karpenko, Charles University, Czech Republic Introduction followed the Thirty Years War. German titles represent one third out of all alchemical books that appeared over The term “alchemy” encompasses a broad spectrum of the whole studied period (4). This is a witness of the activities that appeared in the Hellenistic world in the live interest paid to alchemy in Central Europe; the first centuries of our era and then, through Arabic me- majority of these books are still awaiting scholarly re- diation, reached Latin Europe by the mid 12thcentury. search. Out of numerous attempts to define this science, that Alchemical literature underwent gradual change, proposed by Sheppard (1) appears the most suitable be- being at the beginning often theoretical explanations of cause it includes the two main goals of alchemy: the the composition of matter and recipes for the prepara- enhancement of matter and the improvement of human tion of philosopher’s stone, elixirs, etc. Yet none of these existence. Concerning the former, it should be achieved miracles was effected; no true transmutation of metals by the transmutation of base metals into precious ones, succeeded. An example of the fate of alchemical claims while the second main direction strove for improvement to cure all illnesses was their failure during epidemics of humans by extending their life, the further stage of of plague that broke out in Europe by the mid 14th cen- which was seen as attaining a higher spiritual level. -
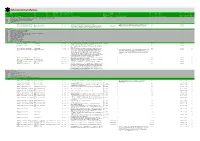
Alchemy Archive Reference
Alchemy Archive Reference 080 (MARC-21) 001 856 245 100 264a 264b 264c 337 008 520 561 037/541 500 700 506 506/357 005 082/084 521/526 (RDA) 2.3.2 19.2 2.8.2 2.8.4 2.8.6 3.19.2 6.11 7.10 5.6.1 22.3/5.6.2 4.3 7.3 5.4 5.4 4.5 Ownership and Date of Alternative Target UDC Nr Filename Title Author Place Publisher Date File Lang. Summary of the content Custodial Source Rev. Description Note Contributor Access Notes on Access Entry UDC-IG Audience History 000 SCIENCE AND KNOWLEDGE. ORGANIZATION. INFORMATION. DOCUMENTATION. LIBRARIANSHIP. INSTITUTIONS. PUBLICATIONS 000.000 Prolegomena. Fundamentals of knowledge and culture. Propaedeutics 001.000 Science and knowledge in general. Organization of intellectual work 001.100 Concepts of science Alchemyand knowledge 001.101 Knowledge 001.102 Information 001102000_UniversalDecimalClassification1961 Universal Decimal Classification 1961 pdf en A complete outline of the Universal Decimal Classification 1961, third edition 1 This third edition of the UDC is the last version (as far as I know) that still includes alchemy in Moreh 2018-06-04 R 1961 its index. It is a useful reference documents when it comes to the folder structure of the 001102000_UniversalDecimalClassification2017 Universal Decimal Classification 2017 pdf en The English version of the UDC Online is a complete standard edition of the scheme on the Web http://www.udcc.org 1 ThisArchive. is not an official document but something that was compiled from the UDC online. Moreh 2018-06-04 R 2017 with over 70,000 classes extended with more than 11,000 records of historical UDC data (cancelled numbers). -

K Or T Á R S M Ű V É S Z E T I F O L Y Ó I R a T • B U Da P E
2013_ 1 Budapest • kortárs művészetifolyóirat ISSN 1216-8890 880 Ft b/2 •• borító tartalom Halász Péter Tamás: Monstrancia 2, 2012, objekt, (pc hulladék; hűtőbordák, alaplapok, stb.) n bicska, p etle en el ge y n „N é : lk s ü ó l” l — k i v M a g k y á a n b r i k e i t n e i v P o n a l 4 társ y: A adalm el at rg á e ta G la i k t ít é ó r m r á ű S v é s inside express z e t 4 • / 1 C i t FKSE_001 t a d e l l Schmied Andi: a r t 2 e . n r 27 m , 2013, installáció T e e d i l n e ä n r u e v n d zali Andre y Imre: A fa rc a: na l h a Im ol el T a T y g e o (t l t u ) 3 d / 6 e C 3 n 3 o s n / v o P y e B t ő e r H l i n u n o r : K ü l d ö m a k é p e m impresszum HU ISSN 1216-8890 Felelős kiadó: Hermann Péter Kiadja és terjeszti a Poligráf Könyvkiadó Levélcím: Balkon, Kállai Ernő Művészeti Alapítvány 2120 Dunakeszi, Keszthelyi István u. 5/a. 1024 Budapest, Fény u. 2. • [email protected] Telefon: +36 27 547 825 • Fax: +36 27 547 826 www.balkon.hu e-mail: [email protected] Főszerkesztő: Hajdu István • [email protected] Szerkesztők: Százados László • [email protected] Szipőcs Krisztina • [email protected] Grafikai terv: Eln Ferenc • [email protected] Fotó: Rosta József • [email protected] ztner Gáb bála: Kozm fis or or os P : A B z ai k rg l éz ik a ik 9 é z ö r S n a y u n v , o m s 2 z a k / 1 e S t t z - a u b n ó i v D e e r z z u s ő m : M / l P a ó g u r a i k a s l u f s e s á s t P í z l l é ü á t l i t e k r s g é jmán jövők sillag Na yi L k és ép / : A c ok m ás lé P se a zl em is e m 0 ó ír zk m ás : S o E n 2 S : s i a P l f s -

Book of Aquarius by Anonymous
THE BOOK OF AQUARIUS BY ANONYMOUS Released: March 20, 2011 Updated: August 16, 2011 The Book of Aquarius By Anonymous. This web edition created and published by Global Grey 2013. GLOBAL GREY NOTHING BUT E-BOOKS TABLE OF CONTENTS 1. The Book Of Aquarius 2. Foreword 3. What Is Alchemy? 4. How Does It Work? 5. The Powers Of The Stone 6. Disbelief 7. Interpretations 8. Obscurity 9. The Secret 10. Yin-Yang 11. Cycles Of Nature 12. Metallic Generation 13. The Emerald Tablet 14. What Is It Made From? 15. The Time 16. The Heat 17. Different Methods 18. Understanding The Writings 19. Overview 20. Apparatus 21. First Part 22. Second Part 23. Black Stage 24. White Stage 25. Fermentation 26. Contradictions 27. Red Stage 28. Multiplication 29. Projection 30. Appearance 31. Everburning Lamps 32. Takwin 33. Religious References 34. Prehistory 35. History Of The Stone 36. Quotes On History 37. Timeline 38. Nicolas Flamel 39. Paracelsus 40. Rosicrucians 41. Francis Bacon 42. Robert Boyle 43. James Price 44. Fulcanelli 45. Where Did They Go? 46. Shambhala 47. Ufos 48. New World Order 49. Mythology 50. Frequency And Planes 51. Universes In Universes 52. The Alchemists' Prophecy 53. Afterword 54. Help 55. Questions And Answers 56. Bibliography 1 The Book of Aquarius By Anonymous 1. The Book Of Aquarius The purpose of this book is to release one particular secret, which has been kept hidden for the last 12,000 years. The Philosophers' Stone, Elixir of Life, Fountain of Youth, Ambrosia, Soma, Amrita, Nectar of Immortality. These are different names for the same thing. -

HISTORY of ALCHEMY Textbook: Linden, Stanton J. 2003. The
Angela Catalina Ghionea Office Hours: W 3:30 – 5:20 pm, UNIV 008 [email protected] and by appointment HISTORY OF ALCHEMY HIST 302 - Fall 2011 MWF 2:30 – 3:20, UNIV 219 I. ASSIGNED READINGS You are required to purchase a textbook and an i>clicker remote for in-class participation. Both the textbook and the i>Clicker remote are available for purchase/rent at any Purdue University Bookstore. Textbook: Linden, Stanton J. 2003. The alchemy reader: from Hermes Trismegistus to Isaac Newton. New York: Cambridge University Press. One copy for the textbook has also been placed on reserve at Hicks Undergraduate Library, and available only at the library (ask Circulation Services at the front desk). Texts are assigned daily to be discussed in class: some available in the textbook, some to be found in pdf. format, posted on the Blackboard LEARN (the online course website). The texts (primary and secondary sources) must be brought in the class. Discussions of these texts count for participation (see the class schedule below). II. OBJECTIVES: The course investigates the history of alchemy and specific non-rational methods employed by many scientists to promote rational discoveries. The course is mainly focused on European alchemy, with few references to Middle Eastern and Arabic alchemy. It spans the period from Antiquity to Renaissance and Early Modern period, and also touches upon nineteenth-century “occult fever” and obsession with alchemy. The course is interdisciplinary and investigates both history of alchemy as well as the more general history -

Encyklopédia Kresťanského Umenia Alfa - Baleka: Prvé Písmeno Gréckej Abecedy a Α; Grécky Písmový Symbol Vyjadrujúci Počiatok; Je Súčasťou Slov Označujúcich Vodu, T.J
Marie Žúborová - Němcová: Encyklopédia kresťanského umenia alfa - Baleka: prvé písmeno gréckej abecedy A α; grécky písmový symbol vyjadrujúci počiatok; je súčasťou slov označujúcich vodu, t.j. počiatok života a silu; najmä v židovstve (pozri Židia, judaizmus) sú mená začínajúce písmenom A početné (Adam apod.); alfa sa často používa na označenie prvého alebo najdôležitejšieho výskytu niečoho (alfa samica); veľké alfa sa zvyčajne ako symbol nepoužíva, pretože je ľahko zameniteľné s písmenom A z latinky; pozri omega Iniciála alfa s maiestas Domini a portrétmi autorov (Beatus Girona, 2. pol. 10.st.) alfa a omega - grécke písmená α ω/A Ω; prvé a posledné písmená gréckej alfabety; pozri Zjavenie Krista, Baleka: alfa a omega sú písmenovým symbolom začiatku a konca, večnosti; vystupujú ako kresťanské symboly Božej nekonečnosti; v zmysle večnosti písmená alfa a omega vkladané do Kristovej svätožiary, najmä pri zobrazeniach na sarkofágoch, ale aj na minciach a hostiách; alfa a omega našli spojitosť so symbolikou orla dvojhlavého > v tejto súvislosti sa vzťahujú ku Kristovi ako k bohu Prvému a Poslednému (koptské, galské a rímske stély) www: písmená alfa a omega sa objavujú v kresťanskom umení od 4.st. a často sú spájané so symbolom Krista (v monogram Krista XP, chrismon, kríž), čím vyjadrujú božskosť Krista a Krista ako Bohočloveka na konci bytia a času sveta; s alfa a omega sa stretávame na epitafoch, kde je súčasťou nápisov, na úžitkových predmetoch ako sú tehly, váze, mince; objavujú sa v sochárstve, mozaike, glyptike a iluminácii; v sochárstve sa objavujú na koptských pohrebných stélach, na zvonoch; v stredoveku sa objavujú na liturgických predmetoch, najmä na paschaloch, na hostiách; v modernom umení symbol alfa a omega mizne, vracia sa až v 19.st. -

Alchemy Diffculties and Dangers in Its Pursuit “Non Ti Fidiare Al Alchemista Povero, O Medico Ammalato."
Theosophical Siftings Alchemy - Difficulties and Dangers in its Pursuit Vol 2, No 1 Alchemy Diffculties and Dangers in its Pursuit “Non ti fidiare al alchemista povero, o medico ammalato." by Parabolanus Reprinted from “Theosophical Siftings” Volume 2 The Theosophical Publishing Society, England Page 1 Theosophical Siftings Alchemy - Difficulties and Dangers in its Pursuit Vol 2, No 1 FROM the very cradle of the greatest civilizations of Antiquity, the precious metals, Gold and Silver, have formed the basis of commercial transactions, facilitated their expansion, and contributed to the mutual intercourse and welfare of mankind. Commerce made easy leads to mutual advancement, civilization, and the spread of knowledge. Gold and Silver must necessarily, at first, have been obtained from the localities where Nature had deposited them. When their great convenience had once established them in general use as the means of obtaining this world's goods — which are considered to be not only the necessaries of life, but also the product of the toil, industry and ingenuity of some classes of men, or of the luxuries and special fruits of one richly endowed soil and climate to be transported to other countries not so favoured — it is evident that the desire of men to possess as much as possible of the precious metals would stimulate some more ambitious and cleverer than their fellows to try to imitate the processes of Nature. From what we now know to be the extreme difficulty of it, we might reasonably suppose that no one, by the exercise of a mere physical intellect, would be able to succeed in doing so. -

Albert Poisson
LA VOCATION DE L’ARBRE D’OR est de partager ses intérêts avec les lecteurs, son admiration pour les grands textes nourrissants du passé et celle aussi pour l’œuvre de contemporains majeurs qui seront probablement davantage appréciés demain qu’aujourd’hui. La belle littérature, les outils de développement personnel, d’identité et de progrès, on les trouvera donc au catalogue de l’Arbre d’Or à des prix résolument bas pour la qualité offerte. LES DROITS DES AUTEURS Cet eBook est sous la protection de la loi fédérale suisse sur le droit d’auteur et les droits voisins (art. 2, al. 2 tit. a, LDA). Il est égale- ment protégé par les traités internationaux sur la propriété indus- trielle. Comme un livre papier, le présent fichier et son image de couverture sont sous copyright, vous ne devez en aucune façon les modifier, les utiliser ou les diffuser sans l’accord des ayants droit. Obtenir ce fichier autrement que suite à un téléchargement après paiement sur le site est un délit. Transmettre ce fichier encodé sur un autre ordinateur que celui avec lequel il a été payé et téléchargé peut occasionner des dommages susceptibles d’engager votre res- ponsabilité civile. Ne diffusez pas votre copie mais, au contraire, quand un titre vous a plu, encouragez-en l’achat : vous contribuerez à ce que les au- teurs vous réservent à l’avenir le meilleur de leur production, parce qu’ils auront confiance en vous. Albert Poisson Nicolas Flamel, histoire et légende © Arbre d’Or, Genève, novembre 2007 http://www.arbredor.com Tous droits réservés pour tous pays PRÉFACE Lorsque, après plusieurs années de recherches laborieuses, nous étions enfin parvenu à retrouver la clef de l’Alchimie, à pouvoir expliquer les obscurs traités des Lulle et des Bacon, à jeter quelque lumière sur cette science aujourd’hui discréditée parce que mal comprise, l’idée se présenta de suite à notre esprit d’exposer l’Alchimie, ses principes, son his- toire, en une série cyclique d’ouvrages, traitant cha- cun de la philosophie hermétique selon quelqu’un de ses différents aspects. -

ALCHEMY 1 Alchemy: Ancient and Modern
ALCHEMY 1 Alchemy: Ancient and Modern Herbert Stanley Redgrove Get any book for free on: www.Abika.com Get any book for free on: www.Abika.com ALCHEMY 2 PREFACE TO THE SECOND EDITION IT is exceedingly gratifying to me that a second edition of this book should be called for. But still more welcome is the change in the attitude of the educated world towards the old-time alchemists and their theories which has taken place during the past few years. The theory of the origin of Alchemy put forward in Chapter I has led to considerable discussion; but whilst this theory has met with general acceptance, some of its earlier critics took it as implying far more than is actually the case. As a result of further research my conviction of its truth has become more fully confirmed, and in my recent work entitled Bygone Beliefs (Rider, 1920), under the title of "The Quest of the Philosopher's Stone," I have found it possible to adduce further evidence in this connection. At the same time, whilst I became increasingly convinced that the main alchemistic hypotheses were drawn from the domain of mystical theology and applied to physics and chemistry by way of analogy, it also became evident to me that the crude physiology of bygone ages and remnants of the old phallic faith formed a further and subsidiary source of alchemistic theory. I have barely, if at all, touched on this Page vi matter in the present work; the reader who is interested will find it dealt with in some detail in "The Phallic Element in Alchemical Doctrine" in my Bygone Beliefs. -

The Alchemy Reader: from Hermes Trismegistus to Isaac Newton'
H-Albion Kusukawa on Linden, 'The Alchemy Reader: From Hermes Trismegistus to Isaac Newton' Review published on Sunday, May 1, 2005 Stanton J. Linden, ed. The Alchemy Reader: From Hermes Trismegistus to Isaac Newton. New York and Cambridge: Cambridge University Press, 2003. xxvii + 260 pp. $79.00 (cloth), ISBN 978-0-521-79234-9; $30.99 (paper), ISBN 978-0-521-79662-0. Reviewed by Sachiko Kusukawa (Trinity College, Cambridge) Published on H-Albion (May, 2005) This is a collection of English translations (previously translated elsewhere) of primary sources relating to alchemy. The collection is divided into three parts (ancient; Islamic and medieval; Renaissance and seventeenth century), each part comprising nine authors. The first part includes excerpts from the works of Hermes Trismegistus The( Emerald Tablet), Plato, Aristotle, Pseudo- Democritus, the anonymous Dialogue of Cleopatra and the philosophers, anonymous recipes, Zosimos of Panopolis, Stephanos of Alexandria, and an anonymous poem; the second part has selections from Khalid ibn Yazid, Jabir ibn Hayyan, Avicenna, Albertus Magnus, Roger Bacon, Nicolas Flamel, Bernard Earl of Trevisan, and George Ripley; the last part contains translations of Paracelsus, Francis Anthony, Michael Sendivogius, Robert Fludd, Gabriel Plattes, John French, George Starkey, Elias Ashmole, Robert Boyle, and Isaac Newton. Each translation is prefaced with a brief biography and description of the excerpts, with a judiciously concise bibliography (a full bibliography is appended at the end of the volume). Although this kind of anthology would never please the purist or the specialist (notable omissions include the writings of Raymond Lull, Arnald of Villanova, and Marsilio Ficino), it is nevertheless helpful to have in one volume so many standard authorities on alchemy and their loci classici. -

Notes and Reviews
NOTES AND REVIEWS Michel Butor and the Tradition of Alchemy Contemporary French writers are currently (and, as usual) engaged in a polemic over the appropriate characteristics of literature. On one side of the fence stand those writers who see literature as a form of communication: form is clearly subservient to the message to be conveyed. Opposing them, are those writers who have surfaced since the zenith of the nouveau roman, and whose ancestry may generally be traced back to the hermeticism of Mallarmé. This latter group, including Jean Ricardou, Philippe Sollers, and Maurice Roche, have in theory and in fact elevated language itself to such awesome stature that it becomes virtually impossible, and in any case pointless, to define the "subject matter" of their works. Somewhere in between these two factions stand a number of relatively well- established authors, including Nathalie Sarraute, Alain Robbe-Grillet (since the Spring Revolution of 1968), and Michel Butor. Butor, as a writer and artist, finds himself in a somewhat awkward position. On the one hand, he subscribes to the theory that his ideal reader must be encouraged and/or coerced to participate actively in the work of art: the reader's effort must be commensurate with that of the author, and Butor finds himself open to accusations of literary elitism and hermeticism. On the other hand, Butor does not accept the human condition as it stands, socio-politically or intellectually. Echoing Rimbaud, Butor insists tfiat life be "changed": "Any literature which does not help us toward Ulis end is eventually, and inevitably, condemned."1 Ironically, a single theme, that of alchemy, suffices to emblematize the contradictory dictates of hermeticism and didacticism.