Marine Organic Geochemistry
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Geochemistry - (2021-2022 Catalog) 1
Geochemistry - (2021-2022 Catalog) 1 GEGN586 NUMERICAL MODELING OF GEOCHEMICAL 3.0 Geochemistry SYSTEMS GEOL512 MINERALOGY AND CRYSTAL CHEMISTRY 3.0 Degrees Offered GEOL513 HYDROTHERMAL GEOCHEMISTRY 3.0 • Master of Science (Geochemistry) GEOL523 REFLECTED LIGHT AND ELECTRON 2.0 MICROSCOPY * • Doctor of Philosophy (Geochemistry) GEOL535 LITHO ORE FORMING PROCESSES 1.0 • Certificate in Analytical Geochemistry GEOL540 ISOTOPE GEOCHEMISTRY AND 3.0 • Professional Masters in Analytical Geochemistry (non-thesis) GEOCHRONOLOGY • Professional Masters in Environmental Geochemistry (non-thesis) GEGN530 CLAY CHARACTERIZATION 2.0 Program Description GEGX571 GEOCHEMICAL EXPLORATION 3.0 The Graduate Program in Geochemistry is an interdisciplinary program * Students can add one additional credit of independent study with the mission to educate students whose interests lie at the (GEOL599) for XRF methods which is taken concurrently with intersection of the geological and chemical sciences. The Geochemistry GEOL523. Program consists of two subprograms, administering two M.S. and Ph.D. degree tracks, two Professional Master's (non-thesis) degree programs, Master of Science (Geochemistry degree track) students must also and a Graduate Certificate. The Geochemistry (GC) degree track pertains complete an appropriate thesis, based upon original research they have to the history and evolution of the Earth and its features, including but not conducted. A thesis proposal and course of study must be approved by limited to the chemical evolution of the crust and -

The Separation of Three Azeotropes by Extractive Distillation by An-I Yeh A
The separation of three azeotropes by extractive distillation by An-I Yeh A thesis submitted in partial fulfillment of the requirement for the degree of Master of Science in Chemical Engineering Montana State University © Copyright by An-I Yeh (1983) Abstract: Several different kinds of extractive distillation agents were investigated to affect the separation of three binary liquid mixtures, isopropyl ether - acetone, methyl acetate - methanol, and isopropyl ether - methyl ethyl ketone. Because of the small size of the extractive distillation column, relative volatilities were assumed constant and the Fenske equation was used to calculate the relative volatilities and the number of minimum theoretical plates. Dimethyl sulfoxide was found to be a good extractive distillation agent. Extractive distillation when employing a proper agent not only negated the azeotropes of the above mixtures, but also improved the efficiency of separation. This process could reverse the relative volatility of isopropyl ether and acetone. This reversion was also found in the system of methyl acetate and methanol when nitrobenzene was the agent. However, normal distillation curves were obtained for the system of isopropyl ether and methyl ethyl ketone undergoing extractive distillation. In the system of methyl acetate and methanol, the relative volatility decreased as the agents' carbon number increased when glycols were used as the agents. In addition, the oxygen number and the locations of hydroxyl groups in the glycols used were believed to affect the values of relative volatility. An appreciable amount of agent must be maintained in the column to affect separation. When dimethyl sulfoxide was an agent for the three systems studied, the relative volatility increased as the addition rate increased. -

Development of a Process for N-Butanol Recovery from Abe
937 A publication of CHEMICAL ENGINEERING TRANSACTIONS VOL. 74, 2019 The Italian Association of Chemical Engineering Online at www.cetjournal.it Guest Editors: Sauro Pierucci, Jiří Jaromír Klemeš, Laura Piazza Copyright © 2019, AIDIC Servizi S.r.l. ISBN 978-88-95608-71-6; ISSN 2283-9216 DOI: 10.3303/CET1974157 Development of a Process for N-Butanol Recovery from Abe Wastewater Streams by Membrane Technology * Marco Stoller , Marco Bravi, Paola Russo, Roberto Bubbico, Barbara Mazzarotta Sapienza University of Rome, Dept. of Chemical Materials Environmental Engineering, Via Eudossiana 18, 00184 Rome, Italy [email protected] The aceton-butyl-ethanolic fermentation process (ABE) is a biotechnological process that leads to the production of acetone, n-butanol and ethanol (ABE compounds) from glucose sources and amides by use of certain biomasses. The process was developed initially during the middle of the last century and suffers from decline due to the greater petrochemical production of products and the lowering of the costs of the sector. Nowadays, the ABE process is regaining great interest because the fraction with the highest concentration, i.e. n-butanol, is an excellent constituent for biofuels. The ABE process has been optimized over time to obtain maximum yields of n-butanol, but the problem of separating and concentrating the butanol in the outlet stream of the ABE process persists. To allow an adequate use, often distillation by use of more columns is required. Moreover, the contained biomasses and suspended solids, in high quantity, must be eliminated, leading to overall high treatment costs. This work will report the main idea and some preliminary experimental results for the development and application of a process based on membrane technologies, to separate and concentrate the butanol from ABE process streams to sensibly reduce the difficulty to perform a final distillation. -

Organic Geochemistry
View metadata, citation and similar papers at core.ac.uk brought to you by CORE provided by ZENODO Organic Geochemistry Organic Geochemistry 37 (2006) 1–11 www.elsevier.com/locate/orggeochem Review Organic geochemistry – A retrospective of its first 70 years q Keith A. Kvenvolden * U.S. Geological Survey, 345 Middlefield Road, MS 999, Menlo Park, CA 94025, USA Institute of Marine Sciences, University of California, Santa Cruz, CA 95064, USA Received 1 September 2005; accepted 1 September 2005 Available online 18 October 2005 Abstract Organic geochemistry had its origin in the early part of the 20th century when organic chemists and geologists realized that detailed information on the organic materials in sediments and rocks was scientifically interesting and of practical importance. The generally acknowledged ‘‘father’’ of organic geochemistry is Alfred E. Treibs (1899–1983), who discov- ered and described, in 1936, porphyrin pigments in shale, coal, and crude oil, and traced the source of these molecules to their biological precursors. Thus, the year 1936 marks the beginning of organic geochemistry. However, formal organiza- tion of organic geochemistry dates from 1959 when the Organic Geochemistry Division (OGD) of The Geochemical Soci- ety was founded in the United States, followed 22 years later (1981) by the establishment of the European Association of Organic Geochemists (EAOG). Organic geochemistry (1) has its own journal, Organic Geochemistry (beginning in 1979) which, since 1988, is the official journal of the EAOG, (2) convenes two major conferences [International Meeting on Organic Geochemistry (IMOG), since 1962, and Gordon Research Conferences on Organic Geochemistry (GRC), since 1968] in alternate years, and (3) is the subject matter of several textbooks. -

Gas Chromatography-Mass Spectroscopy
Gas Chromatography-Mass Spectroscopy Introduction Gas chromatography-mass spectroscopy (GC-MS) is one of the so-called hyphenated analytical techniques. As the name implies, it is actually two techniques that are combined to form a single method of analyzing mixtures of chemicals. Gas chromatography separates the components of a mixture and mass spectroscopy characterizes each of the components individually. By combining the two techniques, an analytical chemist can both qualitatively and quantitatively evaluate a solution containing a number of chemicals. Gas Chromatography In general, chromatography is used to separate mixtures of chemicals into individual components. Once isolated, the components can be evaluated individually. In all chromatography, separation occurs when the sample mixture is introduced (injected) into a mobile phase. In liquid chromatography (LC), the mobile phase is a solvent. In gas chromatography (GC), the mobile phase is an inert gas such as helium. The mobile phase carries the sample mixture through what is referred to as a stationary phase. The stationary phase is usually a chemical that can selectively attract components in a sample mixture. The stationary phase is usually contained in a tube of some sort called a column. Columns can be glass or stainless steel of various dimensions. The mixture of compounds in the mobile phase interacts with the stationary phase. Each compound in the mixture interacts at a different rate. Those that interact the fastest will exit (elute from) the column first. Those that interact slowest will exit the column last. By changing characteristics of the mobile phase and the stationary phase, different mixtures of chemicals can be separated. -

W. M. White Geochemistry Chapter 5: Kinetics
W. M. White Geochemistry Chapter 5: Kinetics CHAPTER 5: KINETICS: THE PACE OF THINGS 5.1 INTRODUCTION hermodynamics concerns itself with the distribution of components among the various phases and species of a system at equilibrium. Kinetics concerns itself with the path the system takes in T achieving equilibrium. Thermodynamics allows us to predict the equilibrium state of a system. Kinetics, on the other hand, tells us how and how fast equilibrium will be attained. Although thermo- dynamics is a macroscopic science, we found it often useful to consider the microscopic viewpoint in developing thermodynamics models. Because kinetics concerns itself with the path a system takes, what we will call reaction mechanisms, the microscopic perspective becomes essential, and we will very often make use of it. Our everyday experience tells one very important thing about reaction kinetics: they are generally slow at low temperature and become faster at higher temperature. For example, sugar dissolves much more rapidly in hot tea than it does in ice tea. Good instructions for making ice tea might then incor- porate this knowledge of kinetics and include the instruction to be sure to dissolve the sugar in the hot tea before pouring it over ice. Because of this temperature dependence of reaction rates, low tempera- ture geochemical systems are often not in equilibrium. A good example might be clastic sediments, which consist of a variety of phases. Some of these phases are in equilibrium with each other and with porewater, but most are not. Another example of this disequilibrium is the oceans. The surface waters of the oceans are everywhere oversaturated with respect to calcite, yet calcite precipitates from sea- water only through biological activity. -

The Role of Geochemistry and Stress on Fracture Development And
Geothermal Technologies Program 2010 Peer Review Public Service of Colorado Ponnequin Wind Farm The Role of Geochemistry and Principal Investigator (Joseph Moore) Stress on Fracture Development Presenter Name (John McLennan) and Proppant Behavior in EGS Organization University of Utah Reservoirs Track Name Reservoir Characterization May 18, 2010 This presentation does not contain any proprietary confidential,1 | US DOE or Geothermalotherwise restricted Program information. eere.energy.gov Timeline DOE Start Date:9/30/2008 DOE Contract Signed: 9/26/2008 Ends 11/30/2011 Project ~40% Complete 2 | US DOE Geothermal Program eere.energy.gov Budget Overview DOE Awardee Total Share Share Project $972,751 $243,188 $1,215,939 Funding FY 2009 $244,869 $107,130 $351,999 FY 2010 $383,952 $64,522 $448,474 FY 2011 $343,930 $71,536 $415,466 DOE Start Date:9/30/2008 DOE Contract Signed: 9/26/2008 Ends 11/30/2011 Project ~40% Complete 3 | US DOE Geothermal Program eere.energy.gov Overview: Barriers and Partners . Barriers to EGS Reservoir Development Addressed: . Reservoir Creation . Long-Term Reservoir and Fracture Sustainability . Zonal Isolation . Partners . Independent Evaluation 4 | US DOE Geothermal Program eere.energy.gov Relevance/Impact of Research Problem . Maximizing initial conductivity of EGS domains . Maintaining long-term conductivity . Facilitate development of extensive stimulated domain via diversion Solution . Proppant placed in fractures Challenge . Proppant behavior at geothermal conditions poorly understood Use of proppant recognized as potential technology under Task “Keep Flow Paths Open” in DOE EGS Technology Evaluation Report 5 | US DOE Geothermal Program eere.energy.gov Relevance/Impact of Research OBJECTIVE Develop Improved Methods For Maintaining Permeable Fracture Volumes In EGS Reservoirs . -

Evaluation of Azeotropic Dehydration for the Preservation of Shrimp. James Edward Rutledge Louisiana State University and Agricultural & Mechanical College
Louisiana State University LSU Digital Commons LSU Historical Dissertations and Theses Graduate School 1969 Evaluation of Azeotropic Dehydration for the Preservation of Shrimp. James Edward Rutledge Louisiana State University and Agricultural & Mechanical College Follow this and additional works at: https://digitalcommons.lsu.edu/gradschool_disstheses Recommended Citation Rutledge, James Edward, "Evaluation of Azeotropic Dehydration for the Preservation of Shrimp." (1969). LSU Historical Dissertations and Theses. 1689. https://digitalcommons.lsu.edu/gradschool_disstheses/1689 This Dissertation is brought to you for free and open access by the Graduate School at LSU Digital Commons. It has been accepted for inclusion in LSU Historical Dissertations and Theses by an authorized administrator of LSU Digital Commons. For more information, please contact [email protected]. This dissertation has been 70-9089 microfilmed exactly as received RUTLEDGE, James Edward, 1941- EVALUATION OF AZEOTROPIC DEHYDRATION FOR THE PRESERVATION OF SHRIMP. The Louisiana State University and Agricultural and Mechanical College, PhJD., 1969 Food Technology University Microfilms, Inc., Ann Arbor, Michigan Evaluation of Azeotropic Dehydration for the Preservation of Shrimp A Dissertation Submitted to the Graduate Faculty of the Louisiana State University and Agricultural and Mechanical College in partial fulfillment of the requirements for the degree of Doctor of Philosophy in The Department of Food Science and Technology by James Edward Rutledge B.S., Texas A&M University, 1963 M.S., Texas A&M University, 1966 August, 1969 ACKNOWLEDGMENT The author wishes to express his sincere appreciation to his major professor, Dr, Fred H. Hoskins, for the guidance which he supplied not only in respect to this dissertation but also in regard to the author’s graduate career at Louisiana State University, Gratitude is also extended to Dr. -
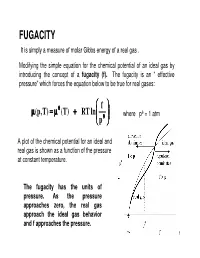
FUGACITY It Is Simply a Measure of Molar Gibbs Energy of a Real Gas
FUGACITY It is simply a measure of molar Gibbs energy of a real gas . Modifying the simple equation for the chemical potential of an ideal gas by introducing the concept of a fugacity (f). The fugacity is an “ effective pressure” which forces the equation below to be true for real gases: θθθ f µµµ ,p( T) === µµµ (T) +++ RT ln where pθ = 1 atm pθθθ A plot of the chemical potential for an ideal and real gas is shown as a function of the pressure at constant temperature. The fugacity has the units of pressure. As the pressure approaches zero, the real gas approach the ideal gas behavior and f approaches the pressure. 1 If fugacity is an “effective pressure” i.e, the pressure that gives the right value for the chemical potential of a real gas. So, the only way we can get a value for it and hence for µµµ is from the gas pressure. Thus we must find the relation between the effective pressure f and the measured pressure p. let f = φ p φ is defined as the fugacity coefficient. φφφ is the “fudge factor” that modifies the actual measured pressure to give the true chemical potential of the real gas. By introducing φ we have just put off finding f directly. Thus, now we have to find φ. Substituting for φφφ in the above equation gives: p µ=µ+(p,T)θ (T) RT ln + RT ln φ=µ (ideal gas) + RT ln φ pθ µµµ(p,T) −−− µµµ(ideal gas ) === RT ln φφφ This equation shows that the difference in chemical potential between the real and ideal gas lies in the term RT ln φφφ.φ This is the term due to molecular interaction effects. -

Coupling Gas Chromatography to Mass Spectrometry
Coupling Gas Chromatography to Mass Spectrometry Introduction The suite of gas chromatographic detectors includes (roughly in order from most common to the least): the flame ionization detector (FID), thermal conductivity detector (TCD or hot wire detector), electron capture detector (ECD), photoionization detector (PID), flame photometric detector (FPD), thermionic detector, and a few more unusual or VERY expensive choices like the atomic emission detector (AED) and the ozone- or fluorine-induce chemiluminescence detectors. All of these except the AED produce an electrical signal that varies with the amount of analyte exiting the chromatographic column. The AED does that AND yields the emission spectrum of selected elements in the analytes as well. Another GC detector that is also very expensive but very powerful is a scaled down version of the mass spectrometer. When coupled to a GC the detection system itself is often referred to as the mass selective detector or more simply the mass detector. This powerful analytical technique belongs to the class of hyphenated analytical instrumentation (since each part had a different beginning and can exist independently) and is called gas chromatograhy/mass spectrometry (GC/MS). Placed at the end of a capillary column in a manner similar to the other GC detectors, the mass detector is more complicated than, for instance, the FID because of the mass spectrometer's complex requirements for the process of creation, separation, and detection of gas phase ions. A capillary column is required in the chromatograph because the entire MS process must be carried out at very low pressures (~10-5 torr) and in order to meet this requirement a vacuum is maintained via constant pumping using a vacuum pump. -

By a 965% ATT'ys
July 19, 1960 A. WATZL ETAL 2,945,788 PROCESS FOR THE PURIFICATION OF DIMETHYLTEREPHTHALATE Filed Nov. 19, l956 AZEOTROPE DISTILLATE CONDENSER CONDENSED WACUUM DISTILLATE DRY DISTILLATION MXTURE N2 COLUMN MPURE - SOD WACUUMFILTER ETHYLENE DMETHYL DMETHYL GLYCOL TEREPHTHALATE TEREPHTHALATE ETHYLENE GLYCOL INVENTORS: ANTON WATZL by aERHARD 965% SIGGEL ATT'YS 2,945,788 Patented July 19, 1960 2 phthalate is immediately suitable for reesterification or Subsequent polycondensation to polyethylene terephthal 2,945,788 ate. There are gained polycondensates of high degree of PROCESS FOR THE PURIFICATION OF viscosity with K values from 50 to 57. DMETHYLTEREPHTHALATE The best mode contemplated for practicing the inven Anton Watz, Kleinwallstadt (Ufr), and Erhard Siggel, tion involves the use of ethylene glycol as the aliphatic Laudenbach (Main), Germany, assignors to Vereinigte glycol. The following is a specific illustration thereof. Glanzstoff-Fabriken A.G., Wuppertal-Elberfeld, Ger Example many Thirty grams of crude dimethylterephthalate are mixed Filed Nov.19, 1956, Ser. No. 622,778 in a flask with 270 grams of ethylene glycol and azeo tropically distilled with the introduction of dry nitrogen 4 Claims. (C. 202-42) in a column of 30 cm. at about 44 Torr (1 Torr equals 1 mm. Hg). The azeotrope goes over at about 120° C. i This invention, in general, relates to production of into a cooled condenser. The dimethylterephthalate dimethylterephthalate and more particularly to the puri 5 separated from the glycol by vacuum filtering can be fication thereof. used immediately for reesterification or for polyconden The purification of dimethylterephthalate can be car sation. This process is illustrated in the flow sheet of ried out either by distillation or by recrystallization from the accompanying drawing. -

Radiogenic Isotope Geochemistry
W. M. White Geochemistry Chapter 8: Radiogenic Isotope Geochemistry CHAPTER 8: RADIOGENIC ISOTOPE GEOCHEMISTRY 8.1 INTRODUCTION adiogenic isotope geochemistry had an enormous influence on geologic thinking in the twentieth century. The story begins, however, in the late nineteenth century. At that time Lord Kelvin (born William Thomson, and who profoundly influenced the development of physics and ther- R th modynamics in the 19 century), estimated the age of the solar system to be about 100 million years, based on the assumption that the Sun’s energy was derived from gravitational collapse. In 1897 he re- vised this estimate downward to the range of 20 to 40 million years. A year earlier, another Eng- lishman, John Jolly, estimated the age of the Earth to be about 100 million years based on the assump- tion that salts in the ocean had built up through geologic time at a rate proportional their delivery by rivers. Geologists were particularly skeptical of Kelvin’s revised estimate, feeling the Earth must be older than this, but had no quantitative means of supporting their arguments. They did not realize it, but the key to the ultimate solution of the dilemma, radioactivity, had been discovered about the same time (1896) by Frenchman Henri Becquerel. Only eleven years elapsed before Bertram Boltwood, an American chemist, published the first ‘radiometric age’. He determined the lead concentrations in three samples of pitchblende, a uranium ore, and concluded they ranged in age from 410 to 535 million years. In the meantime, Jolly also had been busy exploring the uses of radioactivity in geology and published what we might call the first book on isotope geochemistry in 1908.