Downloaded from Consensuspathdb Collection We Defined High-Confidence MTHFD2-Interacting Pro- (
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Upregulation of ALDH1B1 Promotes Tumor Progression in Osteosarcoma
www.impactjournals.com/oncotarget/ Oncotarget, 2018, Vol. 9, (No. 2), pp: 2502-2514 Research Paper Upregulation of ALDH1B1 promotes tumor progression in osteosarcoma Xin Wang1,*, Yan Yu2,*, Yuting He2,*, Qiqing Cai1, Songtao Gao1, Weitao Yao1, Zhiyong Liu1, Zhichao Tian1, Qicai Han3, Weiwei Wang4, Ranran Sun2, Yonggang Luo3 and Chao Li1 1Department of Bone and Soft Tissue, The Affiliated Cancer Hospital of Zhengzhou University, Henan Cancer Hospital, Zhengzhou 450008, China 2Precision Medicine Center, The First Affiliated Hospital of Zhengzhou University, Zhengzhou 450052, China 3Key Laboratory of Clinical Medicine, The First Affiliated Hospital of Zhengzhou University, Zhengzhou 450052, China 4Department of Pathology, The First Affiliated Hospital of Zhengzhou University, Zhengzhou 450052, China *These authors have contributed equally to this work Correspondence to: Chao Li, email: [email protected] Yonggang Luo, email: [email protected] Keywords: osteosarcoma; ALDH1B1; progression; proliferation; metastasis Received: August 21, 2017 Accepted: December 04, 2017 Published: December 20, 2017 Copyright: Wang et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License 3.0 (CC BY 3.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited. ABSTRACT Osteosarcoma (OS) is the most common primary malignant bone tumor in childhood and adolescence with poor prognosis. The mechanism underlying tumorigenesis and development of OS is largely unknown. ALDH1B1 has been reported to involve in many kinds of human cancers and functions as an oncogene, but the role of ALDH1B1 in OS has not been investigated comprehensively. In the present study, we aimed to examine clinical value and biological function of ALDH1B1 in OS. -

Identify Distinct Prognostic Impact of ALDH1 Family Members by TCGA Database in Acute Myeloid Leukemia
Open Access Annals of Hematology & Oncology Research Article Identify Distinct Prognostic Impact of ALDH1 Family Members by TCGA Database in Acute Myeloid Leukemia Yi H, Deng R, Fan F, Sun H, He G, Lai S and Su Y* Department of Hematology, General Hospital of Chengdu Abstract Military Region, China Background: Acute myeloid leukemia is a heterogeneous disease. Identify *Corresponding author: Su Y, Department of the prognostic biomarker is important to guide stratification and therapeutic Hematology, General Hospital of Chengdu Military strategies. Region, Chengdu, 610083, China Method: We detected the expression level and the prognostic impact of Received: November 25, 2017; Accepted: January 18, each ALDH1 family members in AML by The Cancer Genome Atlas (TCGA) 2018; Published: February 06, 2018 database. Results: Upon 168 patients whose expression level of ALDH1 family members were available. We found that the level of ALDH1A1correlated to the prognosis of AML by the National Comprehensive Cancer Network (NCCN) stratification but not in other ALDH1 members. Moreover, we got survival data from 160 AML patients in TCGA database. We found that high ALDH1A1 expression correlated to poor Overall Survival (OS), mostly in Fms-like Tyrosine Kinase-3 (FLT3) mutated group. HighALDH1A2 expression significantly correlated to poor OS in FLT3 wild type population but not in FLT3 mutated group. High ALDH1A3 expression significantly correlated to poor OS in FLT3 mutated group but not in FLT3 wild type group. There was no relationship between the OS of AML with the level of ALDH1B1, ALDH1L1 and ALDH1L2. Conclusion: The prognostic impacts were different in each ALDH1 family members, which needs further investigation. -

Folate Cycle Enzyme MTHFD1L Confers Metabolic Advantages in Hepatocellular Carcinoma
RESEARCH ARTICLE The Journal of Clinical Investigation Folate cycle enzyme MTHFD1L confers metabolic advantages in hepatocellular carcinoma Derek Lee,1 Iris Ming-Jing Xu,1 David Kung-Chun Chiu,1 Robin Kit-Ho Lai,1 Aki Pui-Wah Tse,1 Lynna Lan Li,1 Cheuk-Ting Law,1 Felice Ho-Ching Tsang,1 Larry Lai Wei,1 Cerise Yuen-Ki Chan,1 Chun-Ming Wong,1,2 Irene Oi-Lin Ng,1,2 and Carmen Chak-Lui Wong1,2 1Department of Pathology and 2State Key Laboratory for Liver Research, The University of Hong Kong, Hong Kong, China. Cancer cells preferentially utilize glucose and glutamine, which provide macromolecules and antioxidants that sustain rapid cell division. Metabolic reprogramming in cancer drives an increased glycolytic rate that supports maximal production of these nutrients. The folate cycle, through transfer of a carbon unit between tetrahydrofolate and its derivatives in the cytoplasmic and mitochondrial compartments, produces other metabolites that are essential for cell growth, including nucleotides, methionine, and the antioxidant NADPH. Here, using hepatocellular carcinoma (HCC) as a cancer model, we have observed a reduction in growth rate upon withdrawal of folate. We found that an enzyme in the folate cycle, methylenetetrahydrofolate dehydrogenase 1–like (MTHFD1L), plays an essential role in support of cancer growth. We determined that MTHFD1L is transcriptionally activated by NRF2, a master regulator of redox homeostasis. Our observations further suggest that MTHFD1L contributes to the production and accumulation of NADPH to levels that are sufficient to combat oxidative stress in cancer cells. The elevation of oxidative stress through MTHFD1L knockdown or the use of methotrexate, an antifolate drug, sensitizes cancer cells to sorafenib, a targeted therapy for HCC. -

Supplementary Table S4. FGA Co-Expressed Gene List in LUAD
Supplementary Table S4. FGA co-expressed gene list in LUAD tumors Symbol R Locus Description FGG 0.919 4q28 fibrinogen gamma chain FGL1 0.635 8p22 fibrinogen-like 1 SLC7A2 0.536 8p22 solute carrier family 7 (cationic amino acid transporter, y+ system), member 2 DUSP4 0.521 8p12-p11 dual specificity phosphatase 4 HAL 0.51 12q22-q24.1histidine ammonia-lyase PDE4D 0.499 5q12 phosphodiesterase 4D, cAMP-specific FURIN 0.497 15q26.1 furin (paired basic amino acid cleaving enzyme) CPS1 0.49 2q35 carbamoyl-phosphate synthase 1, mitochondrial TESC 0.478 12q24.22 tescalcin INHA 0.465 2q35 inhibin, alpha S100P 0.461 4p16 S100 calcium binding protein P VPS37A 0.447 8p22 vacuolar protein sorting 37 homolog A (S. cerevisiae) SLC16A14 0.447 2q36.3 solute carrier family 16, member 14 PPARGC1A 0.443 4p15.1 peroxisome proliferator-activated receptor gamma, coactivator 1 alpha SIK1 0.435 21q22.3 salt-inducible kinase 1 IRS2 0.434 13q34 insulin receptor substrate 2 RND1 0.433 12q12 Rho family GTPase 1 HGD 0.433 3q13.33 homogentisate 1,2-dioxygenase PTP4A1 0.432 6q12 protein tyrosine phosphatase type IVA, member 1 C8orf4 0.428 8p11.2 chromosome 8 open reading frame 4 DDC 0.427 7p12.2 dopa decarboxylase (aromatic L-amino acid decarboxylase) TACC2 0.427 10q26 transforming, acidic coiled-coil containing protein 2 MUC13 0.422 3q21.2 mucin 13, cell surface associated C5 0.412 9q33-q34 complement component 5 NR4A2 0.412 2q22-q23 nuclear receptor subfamily 4, group A, member 2 EYS 0.411 6q12 eyes shut homolog (Drosophila) GPX2 0.406 14q24.1 glutathione peroxidase -
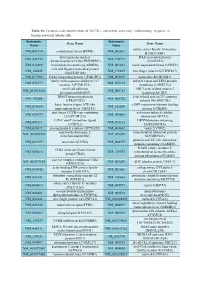
Systematic Name Gene Name Systematic Name Gene Name NM 001710 Complement Factor B(CFB) NM 052831 Solute Carrier Family 18 Member
Table S1: Genome-wide identification of SGLT2i`s interaction with early inflammatory response in human proximal tubular cells. Systematic Systematic Gene Name Gene Name Name Name solute carrier family 18 member NM_001710 complement factor B(CFB) NM_052831 B1(SLC18B1) heterogeneous nuclear DAZ associated protein NM_031372 NM_170711 ribonucleoprotein D like(HNRNPDL) 1(DAZAP1) NM_014299 bromodomain containing 4(BRD4) NM_001261 cyclin dependent kinase 9(CDK9) cilia and flagella associated protein NM_182628 NM_178835 zinc finger protein 827(ZNF827) 100(CFAP100) NM_017906 PAK1 interacting protein 1(PAK1IP1) NM_024015 homeobox B4(HOXB4) family with sequence similarity 167 ankyrin repeat and LEM domain NM_053279 NM_015114 member A(FAM167A) containing 2(ANKLE2) small cell adhesion ARP3 actin related protein 3 NM_001031628 NM_005721 glycoprotein(SMAGP) homolog(ACTR3) TRAF3 interacting protein actin related protein 2/3 complex NM_147686 NM_005720 2(TRAF3IP2) subunit 1B(ARPC1B) basic leucine zipper ATF-like cAMP responsive element binding NM_018664 NM_182898 transcription factor 3(BATF3) protein 5(CREB5) zinc finger CCCH-type containing activation induced cytidine NM_025079 NM_020661 12A(ZC3H12A) deaminase(AICDA) C-X-C motif chemokine ligand DENN domain containing NM_001511 NM_015213 1(CXCL1) 5A(DENND5A) NM_025072 prostaglandin E synthase 2(PTGES2) NM_004665 vanin 2(VNN2) superoxide dismutase 2, mitochondrial ribosomal protein NM_001024465 NM_016070 mitochondrial(SOD2) S23(MRPS23) jumonji and AT-rich interaction NM_033199 urocortin 2(UCN2) NM_004973 -

Transcriptional Silencing of ALDH2 in Acute Myeloid Leukemia Confers a Dependency
bioRxiv preprint doi: https://doi.org/10.1101/2020.10.23.352070; this version posted October 23, 2020. The copyright holder for this preprint (which was not certified by peer review) is the author/funder, who has granted bioRxiv a license to display the preprint in perpetuity. It is made available under aCC-BY 4.0 International license. Transcriptional silencing of ALDH2 in acute myeloid leukemia confers a dependency on Fanconi anemia proteins Zhaolin Yang1, Yiliang Wei1, Xiaoli S. Wu1,2, Shruti V. Iyer1,2, Moonjung Jung3, Emmalee R. Adelman4, Olaf Klingbeil1, Melissa Kramer1, Osama E. Demerdash1, Kenneth Chang1, Sara Goodwin1, Emily Hodges5, W. Richard McCombie1, Maria E. Figueroa4, Agata Smogorzewska3, and Christopher R. Vakoc1,6* 1Cold Spring Harbor Laboratory, Cold Spring Harbor, NY 11724, USA 2Genetics Program, Stony Brook University, Stony Brook, New York 11794, USA 3Laboratory of Genome Maintenance, The Rockefeller University, New York 10065, USA 4Sylvester Comprehensive Cancer Center, Miller School of Medicine, University of Miami, Miami, FL 33136, USA 5Department of Biochemistry and Vanderbilt Genetics Institute, Vanderbilt University School of Medicine, Nashville, TN 37232, USA 6Lead contact *Correspondence: [email protected] Running title: Fanconi anemia pathway dependency in AML Category: Myeloid Neoplasia 1 bioRxiv preprint doi: https://doi.org/10.1101/2020.10.23.352070; this version posted October 23, 2020. The copyright holder for this preprint (which was not certified by peer review) is the author/funder, who has granted bioRxiv a license to display the preprint in perpetuity. It is made available under aCC-BY 4.0 International license. Key Points Dependency on the Fanconi anemia (FA) DNA interstrand crosslink repair pathway is identified in AML. -

Phylogeny and Evolution of Aldehyde Dehydrogenase-Homologous Folate Enzymes
Chemico-Biological Interactions 191 (2011) 122–128 Contents lists available at ScienceDirect Chemico-Biological Interactions journal homepage: www.elsevier.com/locate/chembioint Phylogeny and evolution of aldehyde dehydrogenase-homologous folate enzymes Kyle C. Strickland a, Roger S. Holmes b, Natalia V. Oleinik a, Natalia I. Krupenko a, Sergey A. Krupenko a,∗ a Department of Biochemistry and Molecular Biology, Medical University of South Carolina, Charleston, SC 29425 USA b School of Biomolecular and Physical Sciences, Griffith University, Nathan 4111 Brisbane, Queensland, Australia article info abstract Article history: Folate coenzymes function as one-carbon group carriers in intracellular metabolic pathways. Folate- Available online 6 January 2011 dependent reactions are compartmentalized within the cell and are catalyzed by two distinct groups of enzymes, cytosolic and mitochondrial. Some folate enzymes are present in both compartments Keywords: and are likely the products of gene duplications. A well-characterized cytosolic folate enzyme, FDH Folate metabolism (10-formyltetrahydro-folate dehydrogenase, ALDH1L1), contains a domain with significant sequence Mitochondria similarity to aldehyde dehydrogenases. This domain enables FDH to catalyze the NADP+-dependent 10-Formyltetrahydrofolate dehydrogenase conversion of short-chain aldehydes to corresponding acids in vitro. The aldehyde dehydrogenase-like Aldehyde dehydrogenase Domain structure reaction is the final step in the overall FDH mechanism, by which a tetrahydrofolate-bound formyl group + Evolution is oxidized to CO2 in an NADP -dependent fashion. We have recently cloned and characterized another folate enzyme containing an ALDH domain, a mitochondrial FDH. Here the biological roles of the two enzymes, a comparison of the respective genes, and some potential evolutionary implications are dis- cussed. -

Molecular Signatures Differentiate Immune States in Type 1 Diabetes Families
Page 1 of 65 Diabetes Molecular signatures differentiate immune states in Type 1 diabetes families Yi-Guang Chen1, Susanne M. Cabrera1, Shuang Jia1, Mary L. Kaldunski1, Joanna Kramer1, Sami Cheong2, Rhonda Geoffrey1, Mark F. Roethle1, Jeffrey E. Woodliff3, Carla J. Greenbaum4, Xujing Wang5, and Martin J. Hessner1 1The Max McGee National Research Center for Juvenile Diabetes, Children's Research Institute of Children's Hospital of Wisconsin, and Department of Pediatrics at the Medical College of Wisconsin Milwaukee, WI 53226, USA. 2The Department of Mathematical Sciences, University of Wisconsin-Milwaukee, Milwaukee, WI 53211, USA. 3Flow Cytometry & Cell Separation Facility, Bindley Bioscience Center, Purdue University, West Lafayette, IN 47907, USA. 4Diabetes Research Program, Benaroya Research Institute, Seattle, WA, 98101, USA. 5Systems Biology Center, the National Heart, Lung, and Blood Institute, the National Institutes of Health, Bethesda, MD 20824, USA. Corresponding author: Martin J. Hessner, Ph.D., The Department of Pediatrics, The Medical College of Wisconsin, Milwaukee, WI 53226, USA Tel: 011-1-414-955-4496; Fax: 011-1-414-955-6663; E-mail: [email protected]. Running title: Innate Inflammation in T1D Families Word count: 3999 Number of Tables: 1 Number of Figures: 7 1 For Peer Review Only Diabetes Publish Ahead of Print, published online April 23, 2014 Diabetes Page 2 of 65 ABSTRACT Mechanisms associated with Type 1 diabetes (T1D) development remain incompletely defined. Employing a sensitive array-based bioassay where patient plasma is used to induce transcriptional responses in healthy leukocytes, we previously reported disease-specific, partially IL-1 dependent, signatures associated with pre and recent onset (RO) T1D relative to unrelated healthy controls (uHC). -

Aldehyde Dehydrogenase, Liver Disease and Cancer Wenjun Wang1, Chunguang Wang2, Hongxin Xu1, Yanhang Gao1
Int. J. Biol. Sci. 2020, Vol. 16 921 Ivyspring International Publisher International Journal of Biological Sciences 2020; 16(6): 921-934. doi: 10.7150/ijbs.42300 Review Aldehyde Dehydrogenase, Liver Disease and Cancer Wenjun Wang1, Chunguang Wang2, Hongxin Xu1, Yanhang Gao1 1. Department of Hepatology, The First Hospital of Jilin University, Jilin University, Changchun, Jilin, 130021, China. 2. Department of Thoracic & Cardiovascular Surgery, Second Clinical College, Jilin University, Changchun, 130041, China. Corresponding author: Yanhang Gao, MD., PhD., Department of Hepatology, The First Hospital of Jilin University, Jilin University, Changchun, Jilin, 130021, China. Email: [email protected]. Tel: +86 15804303019; +86 431 81875121; +86 431 81875106; Fax number: 0431-81875106. © The author(s). This is an open access article distributed under the terms of the Creative Commons Attribution License (https://creativecommons.org/licenses/by/4.0/). See http://ivyspring.com/terms for full terms and conditions. Received: 2019.11.20; Accepted: 2020.01.03; Published: 2020.01.22 Abstract Acetaldehyde dehydrogenase 2 (ALDH2) is the key enzyme responsible for metabolism of the alcohol metabolite acetaldehyde in the liver. In addition to conversion of the acetaldehyde molecule, ALDH is also involved in other cellular functions. Recently, many studies have investigated the involvement of ALDH expression in viral hepatitis, alcoholic liver disease (ALD), non-alcoholic fatty liver disease (NAFLD), liver fibrosis, and liver cancer. Notably, ALDH2 expression has been linked with liver cancer risk, as well as pathogenesis and prognosis, and has emerged as a promising therapeutic target. Of note, approximately 8% of the world’s population, and approximately 30-40% of the population in East Asia carry an inactive ALDH2 gene. -

Differentially Expressed Genes Yuki
supplementary Table 1. The differentially expressed genes in F3-treated THP-1 cells after 6 hours. Gene Symbol Chip ID Fold Change Genbank UniGene Description CCL20 205476_at 421.7 NM_004591 Hs.75498 chemokine (C-C motif) ligand 20 CCL4 204103_at 351.6 NM_002984 Hs.75703 chemokine (C-C motif) ligand 4 BCL2A1 205681_at 228.6 NM_004049 Hs.227817 BCL2-related protein A1 ISG20 204698_at 202.1 NM_002201 Hs.459265 interferon stimulated exonuclease gene 20kDa CCL8 214038_at 139.1 AI984980 Hs.271387 chemokine (C-C motif) ligand 8 CXCL1 204470_at 115.1 NM_001511 Hs.789 chemokine (C-X-C motif) ligand 1 (melanoma growth stimulating activity, alpha) IL7R 205798_at 111.6 NM_002185 Hs.362807 interleukin 7 receptor ; interleukin 7 receptor BCL3 204908_s_at 104.2 NM_005178 Hs.31210 B-cell CLL/lymphoma 3 GBP1 202269_x_at 99.99 BC002666 Hs.62661 guanylate binding protein 1, interferon-inducible, 67kDa SLAMF7 219159_s_at 98.3 NM_021181 Hs.517265 SLAM family member 7 CXCL10 204533_at 93.05 NM_001565 Hs.413924 chemokine (C-X-C motif) ligand 10 IL8 211506_s_at 91.31 AF043337 Hs.624 interleukin 8 IL8 202859_x_at 85.26 NM_000584 Hs.624 interleukin 8 THBS1 201110_s_at 84.5 NM_003246 Hs.164226 thrombospondin 1 EBI3 219424_at 82.87 NM_005755 Hs.501452 Epstein-Barr virus induced gene 3 SAT 213988_s_at 76.47 BE971383 Hs.28491 spermidine/spermine N1-acetyltransferase USP18 219211_at 76.22 NM_017414 Hs.38260 ubiquitin specific peptidase 18 LAMP3 205569_at 75.89 NM_014398 Hs.518448 lysosomal-associated membrane protein 3 CCL2 216598_s_at 71.33 S69738 Hs.303649 chemokine -

Mouse Mthfd2l Knockout Project (CRISPR/Cas9)
https://www.alphaknockout.com Mouse Mthfd2l Knockout Project (CRISPR/Cas9) Objective: To create a Mthfd2l knockout Mouse model (C57BL/6J) by CRISPR/Cas-mediated genome engineering. Strategy summary: The Mthfd2l gene (NCBI Reference Sequence: NM_026788 ; Ensembl: ENSMUSG00000029376 ) is located on Mouse chromosome 5. 8 exons are identified, with the ATG start codon in exon 1 and the TAG stop codon in exon 8 (Transcript: ENSMUST00000071652). Exon 2~3 will be selected as target site. Cas9 and gRNA will be co-injected into fertilized eggs for KO Mouse production. The pups will be genotyped by PCR followed by sequencing analysis. Note: Exon 2 starts from about 11.54% of the coding region. Exon 2~3 covers 30.37% of the coding region. The size of effective KO region: ~2156 bp. The KO region does not have any other known gene. Page 1 of 8 https://www.alphaknockout.com Overview of the Targeting Strategy Wildtype allele 5' gRNA region gRNA region 3' 1 2 3 8 Legends Exon of mouse Mthfd2l Knockout region Page 2 of 8 https://www.alphaknockout.com Overview of the Dot Plot (up) Window size: 15 bp Forward Reverse Complement Sequence 12 Note: The 2000 bp section upstream of Exon 2 is aligned with itself to determine if there are tandem repeats. No significant tandem repeat is found in the dot plot matrix. So this region is suitable for PCR screening or sequencing analysis. Overview of the Dot Plot (down) Window size: 15 bp Forward Reverse Complement Sequence 12 Note: The 2000 bp section downstream of Exon 3 is aligned with itself to determine if there are tandem repeats. -

Metabolic Enzyme Expression Highlights a Key Role for MTHFD2 and the Mitochondrial Folate Pathway in Cancer
ARTICLE Received 1 Nov 2013 | Accepted 17 Dec 2013 | Published 23 Jan 2014 DOI: 10.1038/ncomms4128 Metabolic enzyme expression highlights a key role for MTHFD2 and the mitochondrial folate pathway in cancer Roland Nilsson1,2,*, Mohit Jain3,4,5,6,*,w, Nikhil Madhusudhan3,4,5, Nina Gustafsson Sheppard1,2, Laura Strittmatter3,4,5, Caroline Kampf7, Jenny Huang8, Anna Asplund7 & Vamsi K. Mootha3,4,5 Metabolic remodeling is now widely regarded as a hallmark of cancer, but it is not clear whether individual metabolic strategies are frequently exploited by many tumours. Here we compare messenger RNA profiles of 1,454 metabolic enzymes across 1,981 tumours spanning 19 cancer types to identify enzymes that are consistently differentially expressed. Our meta- analysis recovers established targets of some of the most widely used chemotherapeutics, including dihydrofolate reductase, thymidylate synthase and ribonucleotide reductase, while also spotlighting new enzymes, such as the mitochondrial proline biosynthetic enzyme PYCR1. The highest scoring pathway is mitochondrial one-carbon metabolism and is centred on MTHFD2. MTHFD2 RNA and protein are markedly elevated in many cancers and correlated with poor survival in breast cancer. MTHFD2 is expressed in the developing embryo, but is absent in most healthy adult tissues, even those that are proliferating. Our study highlights the importance of mitochondrial compartmentalization of one-carbon metabolism in cancer and raises important therapeutic hypotheses. 1 Unit of Computational Medicine, Department of Medicine, Karolinska Institutet, 17176 Stockholm, Sweden. 2 Center for Molecular Medicine, Karolinska Institutet, 17176 Stockholm, Sweden. 3 Broad Institute, Cambridge, Massachusetts 02142, USA. 4 Department of Systems Biology, Harvard Medical School, Boston, Massachusetts 02115, USA.