Seminiferous Tubules to Epididymis in the Mouse: a Histological and Quantitative Study
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Kidney, Renal Tubule – Dilation
Kidney, Renal Tubule – Dilation Figure Legend: Figure 1 Kidney, Renal tubule - Dilation in a male B6C3F1 mouse from a chronic study. Dilated tubules are noted as tracts running through the cortex and outer medulla. Figure 2 Kidney, Renal tubule - Dilation in a male F344/N rat from a chronic study. Tubule dilation is present throughout the outer stripe of the outer medulla, extending into the cortex. Figure 3 Kidney, Renal tubule - Dilation in a male B6C3F1 mouse from a chronic study. Slight tubule dilation is associated with degeneration and necrosis. Figure 4 Kidney, Renal tubule - Dilation in a male F344/N rat from a chronic study. Tubule dilation is associated with chronic progressive nephropathy. Comment: Renal tubule dilation may occur anywhere along the nephron or collecting duct system. It may occur in focal areas or as tracts running along the entire length of kidney sections (Figure 1). 1 Kidney, Renal Tubule – Dilation Renal tubule dilation may occur from xenobiotic administration, secondary mechanisms, or an unknown pathogenesis (see Kidney – Nephropathy, Obstructive (Figure 2). Dilation may result from direct toxic injury to the tubule epithelium interfering with absorption and secretion (Figure 3). It may also occur secondary to renal ischemia or from prolonged diuresis related to drug administration. Secondary mechanisms of tubule dilation may result from lower urinary tract obstruction, the deposition of tubule crystals, interstitial inflammation and/or fibrosis, and chronic progressive nephropathy (Figure 4). A few dilated tubules may be regarded as normal histologic variation. Recommendation: Renal tubule dilation should be diagnosed and given a severity grade. The location of tubule dilation should be included in the diagnosis as a site modifier. -

Anatomical and Morphological Study of the Kidneys of the Breeding Emu (Dromaius Novaehollandiae)
Turkish Journal of Zoology Turk J Zool (2016) 40: 314-319 http://journals.tubitak.gov.tr/zoology/ © TÜBİTAK Research Article doi:10.3906/zoo-1506-21 Anatomical and morphological study of the kidneys of the breeding emu (Dromaius novaehollandiae) 1, 2 3 2 3 Katarzyna MICHAŁEK *, Danuta SZCZERBIŃSKA , Marta GRABOWSKA , Danuta MAJEWSKA , Maria LASZCZYŃSKA 1 Department of Physiology, Cytobiology, and Proteomics, Faculty of Biotechnology and Animal Husbandry, West Pomeranian University of Technology in Szczecin, Szczecin, Poland 2 Department of Poultry and Ornamental Bird Breeding, West Pomeranian University of Technology in Szczecin, Szczecin, Poland 3 Department of Histology and Developmental Biology, Pomeranian Medical University, Szczecin, Poland Received: 15.06.2015 Accepted/Published Online: 05.01.2016 Final Version: 07.04.2016 Abstract: We analyzed 16 kidneys obtained from 15-year-old emus, Dromaius novaehollandiae. Emus were kept at an experimental farm in Poland. The results showed that each kidney was composed of 3 parts: cranial, medial, and caudal divisions. Histological results demonstrated that the kidneys consisted of 2 zones: the cortex and the medulla. The cortex made up the majority of the kidney, while the medulla formed only a small portion of the organ. Proximal and distal tubules and 2 types of glomeruli (looped and loopless) were localized in the cortex. Each of these glomeruli was characterized by tightly arranged mesangial cells. Proximal and distal tubules had a distinctive simple low cuboidal epithelium. The luminal surface of the proximal tubules had a brush border membrane, formed by numerous microvilli. The renal medulla of emu kidneys formed irregularly positioned characteristic cones of different sizes. -

Male Reproductive System
MALE REPRODUCTIVE SYSTEM DR RAJARSHI ASH M.B.B.S.(CAL); D.O.(EYE) ; M.D.-PGT(2ND YEAR) DEPARTMENT OF PHYSIOLOGY CALCUTTA NATIONAL MEDICAL COLLEGE PARTS OF MALE REPRODUCTIVE SYSTEM A. Gonads – Two ovoid testes present in scrotal sac, out side the abdominal cavity B. Accessory sex organs - epididymis, vas deferens, seminal vesicles, ejaculatory ducts, prostate gland and bulbo-urethral glands C. External genitalia – penis and scrotum ANATOMY OF MALE INTERNAL GENITALIA AND ACCESSORY SEX ORGANS SEMINIFEROUS TUBULE Two principal cell types in seminiferous tubule Sertoli cell Germ cell INTERACTION BETWEEN SERTOLI CELLS AND SPERM BLOOD- TESTIS BARRIER • Blood – testis barrier protects germ cells in seminiferous tubules from harmful elements in blood. • The blood- testis barrier prevents entry of antigenic substances from the developing germ cells into circulation. • High local concentration of androgen, inositol, glutamic acid, aspartic acid can be maintained in the lumen of seminiferous tubule without difficulty. • Blood- testis barrier maintains higher osmolality of luminal content of seminiferous tubules. FUNCTIONS OF SERTOLI CELLS 1.Germ cell development 2.Phagocytosis 3.Nourishment and growth of spermatids 4.Formation of tubular fluid 5.Support spermiation 6.FSH and testosterone sensitivity 7.Endocrine functions of sertoli cells i)Inhibin ii)Activin iii)Follistatin iv)MIS v)Estrogen 8.Sertoli cell secretes ‘Androgen binding protein’(ABP) and H-Y antigen. 9.Sertoli cell contributes formation of blood testis barrier. LEYDIG CELL • Leydig cells are present near the capillaries in the interstitial space between seminiferous tubules. • They are rich in mitochondria & endoplasmic reticulum. • Leydig cells secrete testosterone,DHEA & Androstenedione. • The activity of leydig cell is different in different phases of life. -

Determination of the Elongate Spermatid\P=N-\Sertolicell Ratio in Various Mammals
Determination of the elongate spermatid\p=n-\Sertolicell ratio in various mammals L. D. Russell and R. N. Peterson Department of Physiology, School of Medicine, Southern Illinois University, Carbondale, IL 62901, U.S.A. Summary. Criteria were devised for determining the elongate spermatid\p=n-\Sertolicell ratio in various mammalian species at the electron microscope level. When data from particular species were pooled, the values were: rabbit, 12\m=.\17:1,hamster, 10\m=.\75:1; gerbil, 10\m=.\64:1;rat, 10\m=.\32:1; guinea-pig, 10\m=.\10:1;vole, 9\m=.\75:1;and monkey, 5\m=.\94:1. The elongate spermatid\p=n-\Sertolicell ratio is a measure of the workload of the Sertoli cell and is a prime factor determining their efficiency. The higher the ratio, the higher the sperm output is likely to be per given weight of seminiferous tubule parenchyma for a particular species. Introduction The number of spermatozoa provided in the ejaculate is determined by a number of factors but the major influence is the number of spermatozoa produced in the testis. In mammals that breed continuously testicular sperm production appears to be related to the size of the testis, especially the seminiferous tubule compartment. Here the kinetics of spermatogenesis dictate how many germ cells (spermatogonia) become committed to the spermatogenic process and also the time it takes these germ cells to go through various cell divisions and transformations to become a spermatozoon. The index of sperm production, or the daily sperm production, is expressed as the number of spermatozoa produced per day by the two testes of an individual, whereas the index of efficiency of sperm production is the number of spermatozoa produced per unit weight or volume of testicular tissue (Amann, 1970). -

On the Morphology of the Renal Tubules of Vertebrates
AUTHOR'S ABSTRACT OF THIS PAPER ISSUED BY THE BIBLIOQRAPHIC SERVICE NOVEMBER 10. ON THE MORPHOLOGY OF THE RENAL TUBULES OF VERTEBRATES G. CARL HUBER Department of Anatomy, University of Michigaa TWENTY-TWO FIGURES A comparative study of the renal tubules of the different classes of vertebrates was projected nearly twenty years ago, soon after publishing results of a study on the development and shape of the uriniferous tubules of the higher mammah.1 In this study, as projected, it was purposed to reconstruct the excre- tory tubules of the pronephros and mesonephros of certain of the lower vertebrates, including amphibia, and the metanephric tubules of certain reptiles, birds and mammalia. In the re- construction, by the Born wax plate method, of the pronephric tubules of a larval toad, and mesonephric tubules of an adult frog and the metanephric tubules of certain reptilia no great difficulty was experienced, and such reconstructions were made, somewhat over fifteen years ago in conjunction with Professor Ward J. MacNeal, sometime Instructor in this department. On attempting to reconstruct the metanephric tubules of birds, it was learned after unsuccessful trials that this was beyond the limits of the method, so also with endeavors to reconstruct the metanephric tubules of adult mammalia. The projected study, therefore, was abandoned for a time. The form of the adult, mammalian renal tubule was later ascertained by specially devised methods of teasing.? This special method of teasing has relatively recently been successfully used in an investigation of the form of the metanephric tubule of birds. I am in a position now, therefore, to present figures giving the morphology 1 Huber, G. -

Respiration and Elimination of Nitrogenous Wastes Forms and Functions of Plants and Animals
MODULE - 2 Respiration and Elimination of Nitrogenous Wastes Forms and Functions of Plants and animals 14 Notes RESPIRATION AND ELIMINATION OF NITROGENOUS WASTES Every living organism needs energy to perform various life activities, and the process of respiration fulfils this energy requirement. You have already learnt in the lesson on food and nutrition that animals take in high energy organic molecules in the form of food. During respiration, this food is broken down in the presence of oxygen and energy is released during respiration. Respiration also produces carbon dioxide, a toxic substance which is eliminated from the body. Thus, uptake of oxygen and removal of carbon dioxide is an essential requirement of all animals. At the same time numerous other toxic wastes such as ammonia, and urea are also produced in the tissues during various cellular activities. Such toxic wastes need to be removed from the body. In this lesson you will learn about removal of nitrogenous wastes and maintenance of water and salt balance in the body. OBJECTIVES After completing this lesson you will be able to : z define respiration, breathing, inspiration, expiration and vital capacity; z describe briefly the gaseous exchange in earthworm and cockroach; z describe the parts of respiratory system in the human body and mention their functions; z draw a labeled diagram of human respiratory system; z differentiate between breathing and respiration; and inspiration and expiration; z describe the mechanism of breathing and its regulation; z describe the exchange -

Morphology of the Kidney in a Nectarivorous Bird, the Anna's Hummingbird Calypte Anna
J. Zool., Lond. (1998) 244, 175±184 # 1998 The Zoological Society of London Printed in the United Kingdom Morphology of the kidney in a nectarivorous bird, the Anna's hummingbird Calypte anna G. Casotti1*, C. A. Beuchat2 and E. J. Braun3 1 Department of Biology, West Chester University, West Chester, PA, 19383, U.S.A. 2 Department of Biology, San Diego State University, San Diego, CA, 92182, U.S.A. 3 Department of Physiology, Arizona Health Sciences Center, University of Arizona, P.O. Box 245051, Tucson, AZ, 85724±5051, U.S.A. (Accepted 21 April 1997) Abstract The kidneys of Anna's hummingbird (Calypte anna) differ in several signi®cant ways from those of other birds that have been examined. The kidneys of this nectarivore contain very little medullary tissue; 90% of the total volume of the kidneys is cortical tissue, with medulla accounting for only an additional 2%. More than 99% of the nephrons are the so-called `reptilian type', which lack the loop of Henle. The few looped (`mammalian type') nephrons are incorporated into only a few medullary cones per kidney. The loopless nephrons are similar to those of other birds. However, the looped nephrons differ in that they lack the thin descending limb of the loop of Henle, which is found in other birds and is thought to play an important role in the countercurrent multiplier system in the avian kidney. Instead, the cells of the nephron segment following the pars recta of the proximal tubule resemble those of the thick ascending limb, with the large populations of mitochondria that are typical of transporting epithelia and no reduction in cell height. -
Evaluating and Treating the Kidneys
16_Nephrology.qxd 8/23/2005 10:41 AM Page 451 CHAPTER 16 Evaluating and Treating the Kidneys M. SCOTT ECHOLS, DVM, D ipl ABVP-A vian Renal diseases and their various classifications are well documented in the avian literature. The precise patho- genesis of avian renal disease, however, is not nearly as well described as it is in mammals. Renal disease has been shown to be fairly common in avian species. In studied poultry, as much as 29.6% of all disease condi- tions had abnormal pathology associated with or attrib- utable to renal disorders.20,214,251 Amyloidosis, urate nephrosis and gout were the most common diseases associated with mortality in a 4-year retrospective study conducted at a waterfowl park in Ontario, Canada.210 Thirty-seven percent of all avian cases presenting with renal tissue for histopathological examination, included over a 15-month period at the Schubot Exotic Bird Health Center, had one or more histologically identified kidney lesions.187 Nine of 75 pheasants (Phasianus colchicus) died with nephritis, one or both ureters impacted, and visceral gout in another comprehensive study on avian mortality.186 These case reports and retro- spective studies support the conclusion that renal dis- eases are relatively common and are clinically significant in multiple avian species. When compared to mammalian counterparts, the avian urogenital system has many structural and functional differences, which have been described previously.77,90,118,181,187,227 Differences including gross anatomy, renal portal blood flow and protein waste elimination should be considered when reviewing this chapter, as findings obtained from mammalian studies may not necessarily be applicable to birds. -

PROTEINS of the SEMINIFEROUS TUBULE FLUID in MAN\P=M-\EVIDENCEFOR a BLOOD\P=M-\TESTISBARRIER
PROTEINS OF THE SEMINIFEROUS TUBULE FLUID IN MAN\p=m-\EVIDENCEFOR A BLOOD\p=m-\TESTISBARRIER AARNE I. KOSKIMIES, MARTTI KORMANO and OLOF ALFTHAN Department of Anatomy, University of Helsinki, and Department of Urology of the Second Surgical Clinic, University Central Hospital, Helsinki, Finland (Received 16th December 1971) Summary. Seminiferous tubule fluid was collected by micropuncture from ten human testes immediately after orchidectomy and subjected to high resolution step gradient acrylamide gel electrophoresis. The pro- tein patterns of the fluid were compared with those of serum and intratesticular lymph. The seminiferous tubule fluid always contained a number of proteins not seen in serum or in testicular lymph and a few proteins which were electrophoretically identical with those in serum. The bulk of these relatively weak serum bands consisted of albumin. Disturbance of spermatogenesis did not influence either the appearance of specific proteins or the degree of serum contamination. The present results are interpreted to mean that in man, as in ani- mals, there is an effective blood\p=m-\testisbarrier. The specific proteins of the seminiferous tubules may be elaborated by Sertoli cells. INTRODUCTION The seminiferous tubules secrete a fluid which carries the spermatozoa out of the testis and into the epididymis. The existence of such a fluid has been recognized for some time (von Mihalkovics, 1873; Stieda, 1877), and its circulation was studied microscopically either in normal testes (Rolshoven, 1936) or after ligation of the efferent ducts in experimental animals (Young, 1933). However, it has only recently been shown that the composition of the fluid is unique. This is due both to the existence of a barrier mechanism which prevents the entry of various substances into the seminiferous tubule and to secretory phenomena within the seminiferous epithelium (Setchell, 1971). -

Seminiferous Tubules in Hypophysectomizedrams Treated with Pituitary
Effects of a single brief period of moderate heating of the testes on seminiferous tubules in hypophysectomized rams treated with pituitary extract M. T. Hochereau-de Reviers, A. Locatelli, C. Perreau, C. Pisselet and B. P. Setchell Reproductive Physiology Station, INRA. URA CNRS 1291, Nouzilly 37380, Monnaie, France; and 2On study leave from Department of Animal Sciences, Waite Agricultural Research Institute, The University of Adelaide, Glen Osmond 5064, South Australia, Australia An experiment was conducted to examine the appearance of the seminiferous tubule 20 days after a single exposure of the testes of rams to a scrotal temperature of about 42\s=deg\Cfor 45 min. Ten of the animals were surgically hypophysectomized and five were simultaneously heated; these rams were treated twice a day with ovine pituitary extract to avoid modifi- cations in the negative feedback from the testes to the pituitary and consequent changes in gonadotrophin secretion. Six intact rams (three heated and three unheated) were also studied. The pituitary extract significantly increased the testis weight and spermatogonial multipli- cations from A1 spermatogonia onwards. Twenty days after the heat treatment, testis weight was significantly reduced by heating; both tubular and intertubular tissues were affected. The total length of seminiferous tubules per testis was not modified, whereas the mean seminiferous tubule diameter was significantly reduced after heating. The total number of Sertoli cells per testis was not significantly modified, while their mean cross-sectional nuclear area was significantly reduced by heat treatment. A decrease in the number of all germ cells except A0 spermatogonia, from A1 spermatogonia onwards, was observed. -
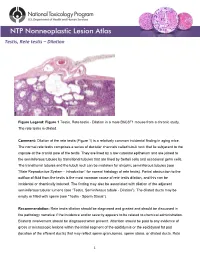
Testis, Rete Testis – Dilation
Testis, Rete testis – Dilation Figure Legend: Figure 1 Testis, Rete testis - Dilation in a male B6C3F1 mouse from a chronic study. The rete testis is dilated. Comment: Dilation of the rete testis (Figure 1) is a relatively common incidental finding in aging mice. The normal rete testis comprises a series of ductular channels called tubuli recti that lie subjacent to the capsule at the cranial pole of the testis. They are lined by a low cuboidal epithelium and are joined to the seminiferous tubules by transitional tubules that are lined by Sertoli cells and occasional germ cells. The transitional tubules and the tubuli recti can be mistaken for atrophic seminiferous tubules (see “Male Reproductive System - Introduction” for normal histology of rete testis). Partial obstruction to the outflow of fluid from the testis is the most common cause of rete testis dilation, and this can be incidental or chemically induced. The finding may also be associated with dilation of the adjacent seminiferous tubular lumens (see “Testis, Seminiferous tubule - Dilation”). The dilated ducts may be empty or filled with sperm (see “Testis - Sperm Stasis”). Recommendation: Rete testis dilation should be diagnosed and graded and should be discussed in the pathology narrative if the incidence and/or severity appears to be related to chemical administration. Bilateral involvement should be diagnosed when present. Attention should be paid to any evidence of gross or microscopic lesions within the initial segment of the epididymis or the epididymal fat pad (location of the efferent ducts) that may reflect sperm granulomas, sperm stasis, or dilated ducts. Rete 1 Testis, Rete testis – Dilation testis dilation may be associated with seminiferous tubular dilation and/or germinal epithelial atrophy, since these may be a consequence of obstruction of fluid outflow. -

The “Road Map”
PRACTICAL ROADMAP MALE REPRODUCTIVE SYSTEM DR N GRAVETT THE TESTIS • Slide 7 Stain: Iron Haematoxylin NOTE: Iron haematoxylin, a blue-black stain demonstrates the chromosomes in the dividing cells of the testis THE TESTIS Connective Tissue Septum These incomplete septae Tunica Albuginea divide the testis into lobes Seminiferous Tubule Interstitial Tissue Loose connective tissue between the seminiferous tubules THE TESTIS Tunica Tunica Albuginea Vasculosa BV Seminiferous Tubule Leydig Cells Blood Vessel (BV) Interstitial Seminiferous Tubule Tissue LEYDIG CELLS Interstitial Tissue BV Seminiferous Tubule NOTE: Leydig cells are endocrine glands and as such are usually located close to blood vessels. These cells are located outside the seminiferous tubules within the loose connective tissue stroma. SEMINIFEROUS TUBULE • Seminiferous Epithelium – Complex Stratified Epithelium consisting of 2 basic cell populations: 1. Sertoli Cells 2. Cells of the Spermatogenic Series: • Spermatogonia • Primary Spermatocyte • Secondary Spermatocyte (Transitory phase: not seen in histological section) • Early Spermatid • Late Spematid SEMINIFEROUS TUBULE Myoid Cell Sertoli Cells Primary Spermatocyte Spermato- gonium Spermato- gonium Lumen Early Spermatids Late Spematids Leydig Cell Spermato- gonium TESTIS AND EPIDIDYMIS • Slide 11 Stain: H&E NOTE: This slide is for ANAT 2020 only Pathway of sperm from point of production to exterior: Seminiferous Tubule Tubuli recti Rete Testes Efferent Ductules Epididymis Vas Deferens Ejaculatory Duct Prostatic Urethra