Equipotential Surfaces and Their Properties 7
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

James Clerk Maxwell
James Clerk Maxwell JAMES CLERK MAXWELL Perspectives on his Life and Work Edited by raymond flood mark mccartney and andrew whitaker 3 3 Great Clarendon Street, Oxford, OX2 6DP, United Kingdom Oxford University Press is a department of the University of Oxford. It furthers the University’s objective of excellence in research, scholarship, and education by publishing worldwide. Oxford is a registered trade mark of Oxford University Press in the UK and in certain other countries c Oxford University Press 2014 The moral rights of the authors have been asserted First Edition published in 2014 Impression: 1 All rights reserved. No part of this publication may be reproduced, stored in a retrieval system, or transmitted, in any form or by any means, without the prior permission in writing of Oxford University Press, or as expressly permitted by law, by licence or under terms agreed with the appropriate reprographics rights organization. Enquiries concerning reproduction outside the scope of the above should be sent to the Rights Department, Oxford University Press, at the address above You must not circulate this work in any other form and you must impose this same condition on any acquirer Published in the United States of America by Oxford University Press 198 Madison Avenue, New York, NY 10016, United States of America British Library Cataloguing in Publication Data Data available Library of Congress Control Number: 2013942195 ISBN 978–0–19–966437–5 Printed and bound by CPI Group (UK) Ltd, Croydon, CR0 4YY Links to third party websites are provided by Oxford in good faith and for information only. -

Electricity Outline
Electricity Outline A. Electrostatics 1. Charge q is measured in coulombs 2. Three ways to charge something. Charge by: Friction, Conduction and Induction 3. Coulomb’s law for point or spherical charges: q1 q2 2 FE FE FE = kq1q2/r r 9 2 2 where k = 9.0 x 10 Nm /Coul 4. Electric field E = FE/qo (qo is a small, + test charge) F = qE Point or spherically symmetric charge distribution: E = kq/r2 E is constant above or below an ∞, charged sheet. 5. Faraday’s Electric Lines of Force rules: E 1. Lines originate on + and terminate on - _ 2. The E field vector is tangent to the line of force + 3. Electric field strength is proportional to line density 4. Lines are ┴ to conducting surfaces. 5. E = 0 inside a hollow or solid conductor 6. Electric potential difference (voltage): ΔV = W/qo We usually drop the Δ and just write V. Sometimes the voltage provided by a battery is know as the electromotive force (emf) ε 7. Potential Energy due to point charges or spherically symmetric charge distribution V= kQ/r 8. Equipotential surfaces are surfaces with constant voltage. The electric field vector is always to an equipotential surface. 9. Emax Air = 3,000,000 N/coul = 30,000 V/cm. If you exceed this value, you will create a conducting path by ripping e-s off air molecules. B. Capacitors 1. Capacitors A. C = q/V; unit of capacitance is the Farad = coul/volt B. For Parallel plate capacitors: V = E d C is proportional to A/d where A is the area of the plates and d is the plate separation. -

Advanced Magnetism and Electromagnetism
ELECTRONIC TECHNOLOGY SERIES ADVANCED MAGNETISM AND ELECTROMAGNETISM i/•. •, / .• ;· ... , -~-> . .... ,•.,'. ·' ,,. • _ , . ,·; . .:~ ~:\ :· ..~: '.· • ' ~. 1. .. • '. ~:;·. · ·!.. ., l• a publication $2 50 ADVANCED MAGNETISM AND ELECTROMAGNETISM Edited by Alexander Schure, Ph.D., Ed. D. - JOHN F. RIDER PUBLISHER, INC., NEW YORK London: CHAPMAN & HALL, LTD. Copyright December, 1959 by JOHN F. RIDER PUBLISHER, INC. All rights reserved. This book or parts thereof may not be reproduced in any form or in any language without permission of the publisher. Library of Congress Catalog Card No. 59-15913 Printed in the United States of America PREFACE The concepts of magnetism and electromagnetism form such an essential part of the study of electronic theory that the serious student of this field must have a complete understanding of these principles. The considerations relating to magnetic theory touch almost every aspect of electronic development. This book is the second of a two-volume treatment of the subject and continues the attention given to the major theoretical con siderations of magnetism, magnetic circuits and electromagnetism presented in the first volume of the series.• The mathematical techniques used in this volume remain rela tively simple but are sufficiently detailed and numerous to permit the interested student or technician extensive experience in typical computations. Greater weight is given to problem solutions. To ensure further a relatively complete coverage of the subject matter, attention is given to the presentation of sufficient information to outline the broad concepts adequately. Rather than attempting to cover a large body of less important material, the selected major topics are treated thoroughly. Attention is given to the typical practical situations and problems which relate to the subject matter being presented, so as to afford the reader an understanding of the applications of the principles he has learned. -

A Selection of New Arrivals September 2017
A selection of new arrivals September 2017 Rare and important books & manuscripts in science and medicine, by Christian Westergaard. Flæsketorvet 68 – 1711 København V – Denmark Cell: (+45)27628014 www.sophiararebooks.com AMPERE, Andre-Marie. Mémoire. INSCRIBED BY AMPÈRE TO FARADAY AMPÈRE, André-Marie. Mémoire sur l’action mutuelle d’un conducteur voltaïque et d’un aimant. Offprint from Nouveaux Mémoires de l’Académie royale des sciences et belles-lettres de Bruxelles, tome IV, 1827. Bound with 18 other pamphlets (listed below). [Colophon:] Brussels: Hayez, Imprimeur de l’Académie Royale, 1827. $38,000 4to (265 x 205 mm). Contemporary quarter-cloth and plain boards (very worn and broken, with most of the spine missing), entirely unrestored. Preserved in a custom cloth box. First edition of the very rare offprint, with the most desirable imaginable provenance: this copy is inscribed by Ampère to Michael Faraday. It thus links the two great founders of electromagnetism, following its discovery by Hans Christian Oersted (1777-1851) in April 1820. The discovery by Ampère (1775-1836), late in the same year, of the force acting between current-carrying conductors was followed a year later by Faraday’s (1791-1867) first great discovery, that of electromagnetic rotation, the first conversion of electrical into mechanical energy. This development was a challenge to Ampère’s mathematically formulated explanation of electromagnetism as a manifestation of currents of electrical fluids surrounding ‘electrodynamic’ molecules; indeed, Faraday directly criticised Ampère’s theory, preferring his own explanation in terms of ‘lines of force’ (which had to wait for James Clerk Maxwell (1831-79) for a precise mathematical formulation). -

Contributions of Maxwell to Electromagnetism
GENERAL I ARTICLE Contributions of Maxwell to Electromagnetism P V Panat Maxwell, one of the greatest physicists of the nineteenth century, was the founder ofa consistent theory ofelectromag netism. However, it must be noted that significant discov eries and intelligent efforts of Coulomb, Volta, Ampere, Oersted, Faraday, Gauss, Poisson, Helmholtz and others preceded the work of Maxwell, enhancing a partial under P V Panat is Professor of standing of the connection between electricity and magne Physics at the Pune tism. Maxwell, by sheer logic and physical understanding University, His main of the earlier discoveries completed the unification of elec research interests are tricity and magnetism. The aim of this article is to describe condensed matter theory and quantum optics. Maxwell's contribution to electricity and magnetism. Introduction Historically, the phenomenon of magnetism was known at least around the 11th century. Electrical charges were discovered in the mid 17th century. Since then, for a long time, these phenom ena were studied separately since no connection between them could be seen except by analogies. It was Oersted's discovery, in July 1820, that a voltaic current-carrying wire produces a mag netic field, which connected electricity and magnetism. An other significant step towards establishing this connection was taken by Faraday in 1831 by discovering the law of induction. Besides, Faraday was perhaps the first proponent of, what we call in modern language, field. His ideas of the 'lines of force' and the 'tube of force' are akin to the modern idea of a field and flux, respectively. Maxwell was backed by the efforts of these and other pioneers. -

09. Fields and Lines of Force. Darrigol (2000), Chap 3
09. Fields and Lines of Force. Darrigol (2000), Chap 3. A. Faraday and Dielectrics • 1835. Insulator in presence of electric source is in a 'state of tension' (essence of electricity). • In presence of electric source, an insulator becomes polarized: positive on one side, negative on the other. • Electric charge belongs to insulators, not conductors. • Insulator = "electric" or "dielectric". Three predictions: 1. No "absolute charge" (charge = "the beginning or ending of polarization"; so, every charge must be related to an opposite charge). 2. Any effect of the composition of the "electric" should prove "that the electricity is related to the electric, not the conductor". 3. "Must try again a very thin plate under induction and look for optical effects." (Kerr effect = change in refractive index of a material in response to an electric field.) Prediction 1: No "absolute charge". • Consider a hollow conductor: If charge derives from the beginning or ending of polarization, there can be no charge inside a conductor, since conductors do not exhibit polarization. • 1835. Inside bottom of copper boiler carries no charge, no matter how electrified the boiler globally is. • 1836. Builds a 12 foot conducting cube with wood, copper, wire, paper, and tin foil and "lived in it" to check internal electric state. • No electricity in "Faraday cage"! • Conclusion: Induction is "illimitable": no global loss of power along a polarized dielectric. • Or: Absolute charge does not exist, insofar as all charge is sustained by induction and is related to another, opposite charge. Prediction 2: Dependence of induced charge on type of dielectric. • Consider device containing two concentric brass spheres. -
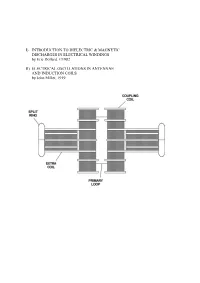
I) Introduction to Dielectric & Magnetic Discharges In
I) INTRODUCTION TO DIELECTRIC & MAGNETIC DISCHARGES IN ELECTRICAL WINDINGS by Eric Dollard, ©1982 II) ELECTRICAL OSCILLATIONS IN ANTENNAE AND INDUCTION COILS by John Miller, 1919 PART I INTRODUCTION TO DIELECTRIC & MAGNETIC DISCHARGES IN ELECTRICAL WINDINGS by Eric Dollard, ©1982 1. CAPACITANCE 2. CAPACITANCE INADEQUATELY EXPLAINED 3. LINES OF FORCE AS REPRESENTATION OF DIELECTRICITY 4. THE LAWS OF LINES OF FORCE 5. FARADAY'S LINES OF FORCE THEORY 6. PHYSICAL CHARACTERISTICS OF LINES OF FORCE 7. MASS ASSOCIATED WITH LINES OF FORCE IN MOTION 8. INDUCTANCE AS AN ANALOGY TO CAPACITANCE 9. MECHANISM OF STORING ENERGY MAGNETICALLY 10. THE LIMITS OF ZERO AND INFINITY 11. INSTANT ENERGY RELEASE AS INFINITY 12. ANOTHER FORM OF ENERGY APPEARS 13. ENERGY STORAGE SPATIALLY DIFFERENT THAN MAGNETIC ENERGY STORAGE 14. VOTAGE IS TO DIELECTRICITY AS CURRENT IS TO MAGNETISM 15. AGAIN THE LIMITS OF ZERO AND INFINITY 16. INSTANT ENERGY RELEASE AS INFINITY 17. ENERGY RETURNS TO MAGNETIC FORM 18. CHARACTERISTIC IMPEDANCE AS A REPRESENTATION OF PULSATION OF ENERGY 19. ENERGY INTO MATTER 20. MISCONCEPTION OF PRESENT THEORY OF CAPACITANCE 21. FREE SPACE INDUCTANCE IS INFINITE 22. WORK OF TESLA, STEINMETZ, AND FARADAY 23. QUESTION AS TO THE VELOCITY OF DIELECTRIC FLUX APPENDIX I 0) Table of Units, Symbols & Dimensions 1) Table of Magnetic & Dielectric Relations 2) Table of Magnetic, Dielectric & Electronic Relations PART II ELECTRICAL OSCILLATIONS IN ANTENNAE & INDUCTION COILS J.M. Miller Proceedings, Institute of Radio Engineers. 1919 1) CAPACITANCE The phenomena of capacitance is a type of electrical energy storage in the form of a field in an enclosed space. This space is typically bounded by two parallel metallic plates or two metallic foils on an interviening insulator or dielectric. -

Michael Faraday: a Virtuous Life Dedicated to Science Citation: F
Firenze University Press www.fupress.com/substantia Historical Article Michael Faraday: a virtuous life dedicated to science Citation: F. Bagnoli and R. Livi (2018) Michael Faraday: a virtuous life dedi- cated to science. Substantia 2(1): 121-134. doi: 10.13128/substantia-45 Franco Bagnoli and Roberto Livi Copyright: © 2018 F. Bagnoli and Department of Physics and Astronomy and CSDC, University of Florence R. Livi. This is an open access, peer- E-mail: [email protected] reviewed article published by Firenze University Press (http://www.fupress. com/substantia) and distribuited under the terms of the Creative Commons Abstract. We review the main aspects of the life of Michael Faraday and some of his Attribution License, which permits main scientific discoveries. Although these aspects are well known and covered in unrestricted use, distribution, and many extensive treatises, we try to illustrate in a concise way the two main “wonders” reproduction in any medium, provided of Faraday’s life: that the son of a poor blacksmith in the Victorian age was able to the original author and source are credited. become the director the Royal Institution and member of the Royal Society, still keep- ing a honest and “virtuous” moral conduct, and that Faraday’s approach to many top- Data Availability Statement: All rel- ics, but mainly to electrochemistry and electrodynamics, has paved the way to the evant data are within the paper and its modern (atomistic and field-based) view of physics, only relying on experiments and Supporting Information files. intuition. We included many excerpts from Faraday’s letters and laboratory notes in order to let the readers have a direct contact with this scientist. -

Appendix A: Fundamentals of Electrostatics
Appendix A: Fundamentals of Electrostatics Antonio Arnau and Tomás Sogorb Departamento de Ingeniería Electrónica, Universidad Politécnica de Valencia A.1 Principles on Electrostatics It is usually attributed to Thales of Miletus (600 B.C.) the knowledge of the property of amber (in Greek elektron) of attracting very light bodies, when rubbed. This phenomenon is called electrification and the cause of the phenomenon electricity. The electric manifestations of electrification phenomena on different materials can be opposite and, in these cases, it is said that both materials have acquired qualities of opposite sign. The quality that a material acquires when electrified is called charge, establishing arbitrarily the sign of this quality in relation to the phenomenon that it manifests. In addition, it could be experimentally demonstrated that the electrified bodies interact with each other, and generate repulsive forces when the charges are of the same sign and, of attraction when they are of opposite sign. The fact that the charge is manifested in two opposite ways involves that if a body has as many positive charges as negative, its external electric manifestations are balanced, and we say that it is electrically neutral. Matter is mainly neutral and it is not common to find bodies whose net charge has a considerable value, therefore, the electrical interactions between bodies are in general quite weak. For this reason, for a material to manifest electric properties it is necessary to subject it to some type of action, for example mechanical, as in the case of amber. This external action produces a loss of balance between the positive and negative charges of the rod and allows the electric interaction with other bodies. -

Proceedings of International Conference on Advanced Research in Mechanical Engineering
Interscience Research Network Interscience Research Network Conference Proceedings - Full Volumes IRNet Conference Proceedings 2-12-2012 Proceedings Of International Conference on Advanced Research in Mechanical Engineering DR. CH R. VIKRAM KUMAR Dept. of Mechanical Engineering N.B.K.R.I.S.T, Vidyanagar Follow this and additional works at: https://www.interscience.in/conf_proc_volumes Part of the Mechanical Engineering Commons Recommended Citation KUMAR, DR. CH R. VIKRAM Dept. of Mechanical Engineering, "Proceedings Of International Conference on Advanced Research in Mechanical Engineering" (2012). Conference Proceedings - Full Volumes. 9. https://www.interscience.in/conf_proc_volumes/9 This Book is brought to you for free and open access by the IRNet Conference Proceedings at Interscience Research Network. It has been accepted for inclusion in Conference Proceedings - Full Volumes by an authorized administrator of Interscience Research Network. For more information, please contact [email protected]. Exprimental Investigation on Effect of Process Parameters In Cold Backward Extrusion P. J. Pawar & D. D. Patil Department of Production Engineering, K.K.W.I.E.E & R, Nashik, India. E-mail : [email protected], [email protected] Abstract - Cold backward extrusion is one of the youngest forming processes. It shows numerous qualitative advantages like high production rate, short piece production time, better surface quality and good static and dynamic quality’s of formed component. Having such enormous application, some of biggest hindrances are power requirement of process to form the product at cold state and non- homogeneous distribution of strain, as result dimensional instability occurs. Aim of study is to find the effect of process parameters in cold backward extrusion process used to manufacture aluminum collapsible tubes. -

Can Magnetic Field Lines Break and Reconnect? Kirk T
Can Magnetic Field Lines Break and Reconnect? Kirk T. McDonald Joseph Henry Laboratories, Princeton University, Princeton, NJ 08544 (December 29, 2015; updated June 23, 2017) 1Problem Following discussion by Giovanelli [1]-[3] and Hoyle [4] of flares near “null points” in the solar magnetic field,1 Dungey (1953) [6] argued that in a region where time-dependent magnetic fields “collide”, field lines near the null point can be said to “break” and “reconnect”. Although the magnetic field energy is negligible very close to a null point, Dungey argued that if the separatrices do not intersect at a right angle, as illustrated in the above left figure, the field energy density is greater in the region between where the angle between the separatrices is obtuse, rather than acute, such that “breaking and reconnection” as in the right figures above is associated with the release of magnetic field energy. This theme was pursued by Sweet [7, 8], and then by Parker [9, 10],2 who considered the figure below in [9]. Discuss “breaking and reconnecting” in a simplified version of Parker’s example, taking this to be a pair of collinear, identical, parallel, “point” magnetic dipoles, m = m zˆ located at (x, y, z)=(0, 0, ±a) in vacuum.3 1The magnetic field is zero at a null point. Such a point in a static electric field is a point of (unstable) equilibrium for electric charges, as considered by Maxwell in arts. 118-121 of his Treatise, and illustrated in his Figs. I-IV at the end of Vol. 1 [5]. 2Sweet and Parker used the term “neutral point” to mean what is called a “null point” here. -

Electricity and Magnetism Electric Field Contents
Electricity and Magnetism Electric Field Contents 1 Electric charge 1 1.1 Overview ............................................... 2 1.2 Units ................................................. 4 1.3 History ................................................ 4 1.4 Static electricity and electric current ................................. 4 1.4.1 Electrification by friction ................................... 5 1.5 Properties ............................................... 6 1.6 Conservation of electric charge .................................... 7 1.7 See also ................................................ 7 1.8 References .............................................. 7 1.9 External links ............................................. 7 2 Electrostatic induction 8 2.1 Explanation .............................................. 8 2.2 Charging an object by induction ................................... 9 2.3 The electrostatic field inside a conductive object is zero ........................ 10 2.4 Induced charge resides on the surface ................................. 10 2.5 Induction in dielectric objects ..................................... 10 2.6 Notes ................................................. 12 2.7 External links ............................................. 12 3 Coulomb’s law 13 3.1 History ................................................ 13 3.2 The law ................................................ 14 3.2.1 Units ............................................. 16 3.2.2 Electric field .......................................... 16