Directions for Use Euro-Med®
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

SURGICAL INSTRUMENTS Veterinarians Are the Doctors Specializing in the Health of Animals
SURGICAL INSTRUMENTS Veterinarians are the doctors specializing in the health of animals. They do the necessary surgical operations and care for the well-being of the animal creatures. The very basic thing they need in a certain operation and care are the veterinary instruments. This will serve as the main allay of every veterinarian in providing care. (1) What are surgical instruments? Surgical instruments are essentially gadgets planned in an uncommon manner to perform particular capacities amid a surgical operation to improve viability and accomplishment of the surgery. (1) 4 Basic types of surgical instruments Surgical instruments are specially designed tools that assist health care professionals car- ry out specific actions during an operation. Most instruments crafted from the early 19th century on are made from durable stainless steel. Some are designed for general use, and others for spe- cific procedures. There are many surgical instruments available for almost any specialization in medicine. There are precision instruments used in microsurgery, ophthalmology and otology. Most surgical instruments can be classified into these 4 basic types: Cutting and Dissecting – these instruments usually have sharp edges or tips to cut through skin, tissue and suture material. Surgeons need to cut and dissect tissue to explore irregular growths and to remove dangerous or damaged tissue. These instruments have single or double razor- sharp edges or blades. Nurses need to be very careful to avoid injuries, and regularly inspect these instruments before using, for re-sharpening or replacement. 11 Iris Scissors 2016 – 1 – LV01-KA202 – 022652 This project is funded by the European Union Clamping and Occluding – are used in many surgical procedures for compressing blood vessels or hollow organs, to prevent their contents from leaking. -

The World of SURGICAL INSTRUMENTS
Textbook Preview The World of SURGICAL INSTRUMENTS The Definitive Inspection Textbook 501 full color pages 1,198 high resolution photos Glossy, hard cover Lay flat design by RICK SCHULTZ SCISSORS Frequently Asked Questions Q: Do all scissors go dull? A: Yes. Every scissor goes dull no matter what size, specialty, manufacturer, or design. Q: Does sterilization dull a scissor? A: Generally, no. However, when old autoclaves produce dirty steam, the scissor blade edges can become stained, which can cause the scissor not to cut. Q: Can all scissors be resharpened? A: Yes. Every scissor can be resharpened. Make sure the repair vendor is properly trained, especially on SuperCut scissors (black-handled). Q: How often should scissors be tested? A: Scissors should be tested 1 to 2 times per week. The proactive approach is picking 2 days per week as scissor testing days. Using an instrument tracking system will allow the facility to track sharpening frequency. Eventually, a large percentage of scissors will be sharp. Q: Is the scissor testing standard red and yellow scissor test material? A: Yes. The industry standard is to use red scissor test material for scissors longer than 41⁄2" in overall length and yellow scissor test material for scissors that measure 41⁄2" and shorter. Yellow scissor test material is used on 41⁄2" scissors. Q: Do all repair technicians know how to sharpen scissors? A: No. Experience and proper training is key, and many times repair technicians are learning on your expensive instrument inventory. The hospital should verify how many months of training/employment the repair technician has. -

Vitreo Retina Surgical Instruments Catalog 2014
BR554-14 General Information Vitreo Retina Instruments: Trocar Set WARRANTY/GUARANTEE All ASICO ophthalmic instruments are unconditionally guaranteed for life against manufacturing defects in material and workmanship when used for their intended surgical purpose and cared for in accordance with recommended procedure. In the event any ASICO instrument should fail as a result of a manufacturing defect in material or workmanship, it will be replaced or repaired at no charge. TERMS OF PAYMENT Invoices are payable on receipt. If payment is received after the initial 30-day period, a finance charge of 1.5% for an annual rate of 18% will be charged. SHIPPING Shipments of surgical instruments are made via FedEx Second Day Air. Shipment can also be made via UPS or DHL upon customer request, with a valid shipping account number for requested shipping vendor. If customer uses their shipping account number a handling fee will still apply to the order. ORDERS Orders can be placed through our toll-free customer service line, 1-800-628-2879, by fax, by calling 1-630-986-8032 or by emailing [email protected]. PRICES Prices are in US Dollars and are subject to change without notice. RETURN POLICY All products returned to ASICO must be accompanied by a return authorization number. This number can be obtained by contacting ASICO by phone or email. Merchandise should be carefully packaged, shipped prepaid, insured and mailed to the Westmont office. Each shipment must be accompanied by a document showing the full name and address of a sender, list of items, and the reason for return. -

Handling of Instruments
Limbs & Things TM learning online Handling of Instruments Overview If you are new to suturing, you will need to learn to recognize each of the instruments you are going to use, understand their function and practise the basic techniques of using them. 1 Scalpel A scalpel is a razor-edged blade on a handle. There are two types of surgical scalpel: reusable and disposable. Reusable scalpels consist of a blade that is replaced after every use, attached to a stainless steel handle that can be sterilised and re-used multiple times. In a hospital setting, this type is more likely to be used in order to reduce waste and to allow doctors to work with a variety of blades and handle sizes. Disposable surgical scalpels are usually single-piece with a plastic handle. This is the type provided in our Hands-on Kit for practice. Although you will use the same scalpel multiple times for practice, in a clinical setting you would dispose of the entire scalpel after a single use. 1.1 Principles A scalpel is essential for incising the skin and for sharp dissection. Held flat, it can also be useful for carefully undermining the skin edge to relieve tension. A razor edged blade engages over a flange on the scalpel handle. Several sizes of scalpel handle are available and size 3 is appropriate for most purposes. Each handle can be fitted with disposable blades of different shapes. The scalpel can be held in one of two ways: - For making large incisions e.g. laparotomy, and subcutaneous fat dissection, hold the scalpel like a table knife, with your index finger guiding the blade. -
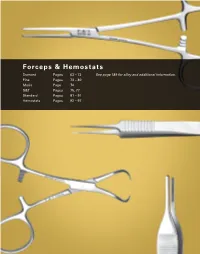
Forceps & Hemostats
Forceps & Hemostats Dumont Pages 63 – 72 See page 189 for alloy and additional information. Fine Pages 73 – 80 Moria Page 74 S&T Pages 75, 77 Standard Pages 81 – 91 Hemostats Pages 92 – 97 DUMONT® FORCEPS Dumont #5SF Dumont #5 Dumont #5 Dumont #5CO The finest tips ever Fine Tips Ceramic Coated These forceps have been produced by Dumont. Finished under magnification and These forceps are non-reflective. honed down to make this the polished on the inside, the tips The scratch and corrosion resistant lightest and most precise close flat to 3 mm and are less likely coating guarantees durability. Dumont forceps available. to open under excess pressure. FORCEPS For information on Dumont alloys and tip styles, see page 189. Super fine tip Biology tip Biology tip Biology tip 0.025 x 0.005 mm 0.05 x 0.01 mm 0.05 x 0.01 mm 0.05 x 0.01 mm Inox Inox Ceramic coated inox Dumostar 11 cm 11 cm 11 cm 11 cm No. 11252-00 No. 11254-20 No. 11252-50 No. 11295-20 63 FINESCIENCE.COM · MOST ITEMS SHOWN ACTUAL SIZE DUMONT® FORCEPS Dumont Micro-Blunted, Atraumatic Tipped Forceps The sides of these forceps are distinctively ribbed to provide a better holding surface for maximum control of the instrument. The shanks are arched toward each other to transmit more holding strength to the points. The smooth inside surfaces at the tips will close flat to 5 mm with gentle pressure, increasing the total holding area considerably. The microscopically matched tips are gently blunted to prevent tissue puncture and are recommended as micro needle holders. -

VATS Instruments
VATS Instruments Video-Assisted Thoracoscopic Surgery Surgical Speciality Products VATS Instruments Video-Assisted Thoracoscopic Surgery Forceps / Clamps | Diameter 7 mm | Usable length 25 cm STANDARD INSTRUMENT - COMPLETE Description FOERSTER LUNG GRASPER 25 cm 15 mm oval ring jaws, 15° angled, 63 mm jaw length, Forceps/Clamps double action ¹⁄1 FC810R 25 cm TISSUE GRASPING FORCEPS 30° angled, 65 mm jaw length, single action ¹⁄1 FC811R 25 cm MARYLAND DISSECTING FORCEPS curved, 21 mm jaw length, double action ¹⁄1 FC812R 25 cm DISSECTING FORCEPS straight, 25 mm jaw length, double action ¹⁄1 FC813R 2 SINGLE COMPONENTS Jaw Insert Insulated outer tube Handle Forceps/Clamps ½ FC860R without ratchet ½ FC800R FC861R FC808P with ratchet, disengageable ½ FC801R FC862R ½ FC863R 3 VATS Instruments Video-Assisted Thoracoscopic Surgery Forceps / Clamps | Diameter 7 mm | Usable length 25 cm STANDARD INSTRUMENT - COMPLETE Description OVERHOLT DISSECTING FORCEPS 90° 65 mm jaw length, double action Forceps/Clamps 25 cm ¹⁄1 FC814R OVERHOLT DISSECTING FORCEPS 90° delicate, 64 mm jaw length, double action 25 cm ¹⁄1 FC815R 25 cm OVERHOLT DISSECTING FORCEPS 60° 40 mm jaw length, double action ¹⁄1 FC816R BIOPSY FORCEPS 30° curved, 14 mm jaw length, double action ¹⁄1 FC820R 4 SINGLE COMPONENTS Jaw Insert Insulated outer tube Handle Forceps/Clamps ½ FC864R without ratchet ½ FC800R FC865R FC808P with ratchet, disengageable ½ FC801R FC866R ½ FC870R 5 VATS Instruments Video-Assisted Thoracoscopic Surgery Forceps / Clamps | Diameter 7 mm | Usable length 25 cm STANDARD -

ICD-10-PCS Reference Manual
ICD-10-PCS Reference Manual 3 Table of Contents Preface ............................................................................................ xi Manual organization ................................................................................................................. xi Chapter 1 - Overview ......................................................................................................... xi Chapter 2 - Procedures in the Medical and Surgical section ............................................. xi Chapter 3 - Procedures in the Medical and Surgical-related sections ............................... xi Chapter 4 - Procedures in the ancillary sections ............................................................... xii Appendix A - ICD-10-PCS definitions ................................................................................ xii Appendix B - ICD-10-PCS device and substance classification ........................................ xii Conventions used ..................................................................................................................... xii Root operation descriptions ............................................................................................... xii Table excerpts ................................................................................................................... xiii Chapter 1: ICD-10-PCS overview ......................................................... 15 What is ICD-10-PCS? ............................................................................................................. -

Instruments for Cardiac and Vascular Surgery
Instruments for Cardiac and Vascular Surgery Aesculap Surgical Specialty Products - Cardiothoracic Instruments for Cardiac and Vascular Surgery OPERATE WITH GREATER PRECISION At Aesculap, we understand your practice faces evolving challenges from a rapidly aging patient population and increasingly complex diseases. For that reason, we are committed to delivering innovative cardiothoracic technologies, designed and manufactured with the same high standard of quality we have maintained for over 150 years. Our focus on solutions for minimally-invasive cardiothoracic surgery is driven by the desire to improve patient outcomes. In addition to our portfolio of cutting-edge technologies for beating heart coronary surgery, we offer a broad line of specialized instruments and retractors designed to meet the demanding needs of today’s cardiovascular, vascular and thoracic surgeons. In this catalog, you will find a select range of instruments that are commonly used in cardiac and vascular surgery. As the world’s largest instrument manufacturer, we are proud of our strict quality standards and meticulous workmanship that allow you to address challenging cardiac and vascular procedures with greater precision. The working surfaces of Aesculap DUROGRIP™ The non-reflective TITANIUM instruments the jaws of all needle holders have NOIR™ coating is six provide incomparable Dia Dust™ instruments jaws with high quality times as hard as normal operating sensitivity are coated with a fine tungsten carbide inserts. instrument steel, through light-weight layer of diamond dust, The smooth jaw surface providing increased handling. With a non- which results in a hard as well as the 0.2 mm resistance against both reflective finish and surface with high wear micro serration guarantee wear and corrosion. -

Instruments & Tools
Sterilzed tools at your fingertips Instruments & Tools Forceps Save Time & Money Scalpels • Ready-to-use, pre-packaged instruments, tools and sets. • Eliminates sterilization step in your lab. Instrument • Avoid exposure to infectious disease with disposable sets. Sets • Economical sterile and non-sterile options available. Tissue Grinders Reduce Effort & Frustration Mini Grip-it • Complete instrument sets ready when busy labs need them fast. • Customize a set for your lab’s specific needs with Autoclave appropriately small minimums. Bags • Innovative tools make time-intensive lab tasks easier. For more information see the catalog on our website. See product listing on reverse side. scientificdevice.com [email protected] call us 847.803.9495 Fine-tip Forceps Sterile Instrument Set • Easy precision with small specimens • 1 stainless steel 11mm forceps • 4” long autoclavable plastic • 1 rigid plastic scalpel holder with #11 blade • Sterile option also available • 1 stainless steel pointed scissor 9005-50 Fine-tip Forceps NON-sterile – 50/pkg • Autoclavable scissors and forceps 9005-S Fine-tip Forceps Sterile – 100/pkg 903 Sterile Instrument Sets – 12 sets/pkg 900 Forceps 4.5” Stainless Steel Sterile – 25/pkg 9003 Sterile Scalpel #11 Blade – 100/pkg 900-100 Forceps 4.5” Stainless Steel Sterile – 100/pkg 900-NS Forceps 4.5” Stainless Steel NON-sterile – 100/pkg Sterile Instrument Set with Sterile Dissecting Board Rounded-tip Forceps • 1 fine-tipped forcep • 1 rigid plastic scalpel holder with #11 blade • Grooved tips grip samples -

Obstetrical Forceps: History, Mystery, and Morality
The Texas Medical Center Library DigitalCommons@TMC John P. McGovern Historical Collections and Houston History of Medicine Lectures Research Center 9-7-2011 Obstetrical Forceps: History, Mystery, and Morality Ronald L. Young Baylor College of Medicine Follow this and additional works at: https://digitalcommons.library.tmc.edu/homl Recommended Citation Citation Information:Young, Ronald L., "Obstetrical Forceps: History, Mystery, and Morality" (2011). DigitalCommons@TMC, John P. McGovern Historical Collections and Research Center, Houston History of Medicine Lectures. Paper 13. https://digitalcommons.library.tmc.edu/homl/13 This Article is brought to you for free and open access by the John P. McGovern Historical Collections and Research Center at DigitalCommons@TMC. It has been accepted for inclusion in Houston History of Medicine Lectures by an authorized administrator of DigitalCommons@TMC. For more information, please contact [email protected]. Houston History of Medicine September 7, 2011 Robert Rakel, M.D. Welcome to our first program for this academic year for the Houston History of Medicine Society. As you all know, you can find everything on our website at www.hhom.org. We are fortunate today to have one of our favorite speakers. Dr. Ron Young is the head of the Department of Gynecology in the Baylor Department of OB/GYN. He did his basic training in Basel, Switzerland. I just need to tell you my favorite Switzerland story, if I may. A group of us were there for a meeting. We went to dinner and walked out from dinner, and there were no cabs around, so I said, “I will walk and find one. -

Dissection Instruments FORCEPS
Dissection Instruments FORCEPS WM0413S3 Custom Embedding Forceps: 13cm, Curved, Standard Grade $16.67 Custom Embedding Forceps: 15cm, Curved, Standard Grade, fine tip, WM0415S3 $17.16 smooth Custom Embedding Forceps: 13cm, Angled Curve, Standard Grade, fine WM1413S3 $16.67 tip, smooth Custom Embedding Forceps: 15cm, Angled Curve, Standard Grade, fine WM1415S3 $17.16 tip, smooth Custom Embedding Forceps: 18cm, Curved, Standard Grade, fine tip, WM0418S3 $18.11 smooth WM0202S3 Standard Forceps: 11.5cm, Straight, Standard Grade, broad tip, serrated $10.80 WM0205S3 Standard Forceps: 16cm, Straight, Standard Grade, broad tip, serrated $15.80 WM0220S3 Standard Forceps: 20cm, Standard Grade, broad tip, serrated $17.28 To order contact PathSUPPLY, Inc. at 800-631-3556 or [email protected] or fax 702-921-7129. For orders of 10 or more instruments contact us for special pricing. PathSUPPLY is a veteran owned small business. Dissection Instruments WM0208S3 Standard Forceps: 25cm, Straight, Standard Grade, broad tip, serrated $23.76 WM0209S3 Standard Forceps: 30cm, Straight, Standard Grade, broad tip, serrated $30.36 Custom Embedding Forceps: 13cm, Straight, Standard Grade, fine tip, WM0313S3 $15.12 smooth Custom Embedding Forceps: 15cm, Straight, Standard Grade, fine tip, WM0315S3 $15.84 smooth Custom Embedding Forceps: 18cm, Straight, Standard Grade, fine tip, WM0318S3 $16.56 smooth WM0405S3 Embedding Forceps: 16cm, Straight, Standard Grade, broad tip, smooth $19.44 WM0938S3 Ergonomic Forceps, 7” (18 cm), 1:2 teeth, straight, Premium Grade $60.00 Ergonomic Forceps, 7” (18 cm), 1 mm smooth tip, straight, Premium WM0948S3 $60.00 Grade To order contact PathSUPPLY, Inc. at 800-631-3556 or [email protected] or fax 702-921-7129. -

Surgical Instruments
BR SURGICAL • SURGICAL PROCEDURE EXPERTS INSTRUMENTS | SETS ENDOSCOPIC VIDEO EQUIPMENT OVER 20,000 SURGICAL INSTRUMENTS & PROCEDURE SETS ENDOSCOPIC VIDEO EQUIPMENT & RELATED MEDICAL DEVICES SURGICAL BR SURGICAL, LLC • 3500 Beachwood Court • Suite 107 • Jacksonville, Florida 32224 Office: (904) 642-1366 • Fax: (904) 642-1368 • Toll Free: (888) 642-1366 INSTRUMENTS www.brsurgical.com OVER 20,000 SURGICAL INSTRUMENTS & PROCEDURE SETS ENDOSCOPIC VIDEO EQUIPMENT & RELATED MEDICAL DEVICES BR SURGICAL, LLC F Precision crafted German stainless steel instruments F Surgical instrument pattern is equal to or better than industry standard F All instruments put through our stringent inspection process to ensure consistent high quality F Continuous quality improvements F INDUSTRY BEST WARRANTY Surgical instruments are guaranteed to be free of defects in materials and workmanship for the life of the instrument F FREE LIFETIME SHARPENING on Gold Series biopsy punches Customer sends the biopsy punch with return address to BR Surgical and we take care of the rest F Customer issues handled to their complete satisfaction Scan this QR Code with your smartphone or tablet and connect with us online! BR SURGICAL, LLC • 3500 Beachwood Court • Suite 107 • Jacksonville, Florida 32224 Office: (904) 642-1366 • Fax: (904) 642-1368 • Toll Free: (888) 642-1366 www.brsurgical.com CONTENTS Instrument Care . .2 Repair Service . 3 Limited Return Policy . .3 Custom Instruments . .3 Warranty Cross Reference Information . 3 Endoscope Cleaning & Sterilizing Information . .4 Hysteroscopy Video System . .6 BR Surgical, LLC Hysteroscopy Accessories . .7 instruments are Cystoscopy Video System . 8 ENT/Sinus Video System . 9 guaranteed to be Flexible Rhinolaryngoscope Video System . 10 free of defects Flexible Rhinolaryngoscope .