Achieving Safe DICOM Software in Medical Devices
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Artificial Intelligence in Health Care: the Hope, the Hype, the Promise, the Peril
Artificial Intelligence in Health Care: The Hope, the Hype, the Promise, the Peril Michael Matheny, Sonoo Thadaney Israni, Mahnoor Ahmed, and Danielle Whicher, Editors WASHINGTON, DC NAM.EDU PREPUBLICATION COPY - Uncorrected Proofs NATIONAL ACADEMY OF MEDICINE • 500 Fifth Street, NW • WASHINGTON, DC 20001 NOTICE: This publication has undergone peer review according to procedures established by the National Academy of Medicine (NAM). Publication by the NAM worthy of public attention, but does not constitute endorsement of conclusions and recommendationssignifies that it is the by productthe NAM. of The a carefully views presented considered in processthis publication and is a contributionare those of individual contributors and do not represent formal consensus positions of the authors’ organizations; the NAM; or the National Academies of Sciences, Engineering, and Medicine. Library of Congress Cataloging-in-Publication Data to Come Copyright 2019 by the National Academy of Sciences. All rights reserved. Printed in the United States of America. Suggested citation: Matheny, M., S. Thadaney Israni, M. Ahmed, and D. Whicher, Editors. 2019. Artificial Intelligence in Health Care: The Hope, the Hype, the Promise, the Peril. NAM Special Publication. Washington, DC: National Academy of Medicine. PREPUBLICATION COPY - Uncorrected Proofs “Knowing is not enough; we must apply. Willing is not enough; we must do.” --GOETHE PREPUBLICATION COPY - Uncorrected Proofs ABOUT THE NATIONAL ACADEMY OF MEDICINE The National Academy of Medicine is one of three Academies constituting the Nation- al Academies of Sciences, Engineering, and Medicine (the National Academies). The Na- tional Academies provide independent, objective analysis and advice to the nation and conduct other activities to solve complex problems and inform public policy decisions. -

Programové Vybavení Pro Provoz Nukleární Medicíny Software
ČESKÉ VYSOKÉ UČENÍ TECHNICKÉ V PRAZE FAKULTA BIOMEDICÍNSKÉHO INŽENÝRSTVÍ Katedra biomedicínské techniky Programové vybavení pro provoz nukleární medicíny Software Systems for Nuclear Medicine Diplomová práce Studijní program: Biomedicínská a klinická technika Studijní obor: Systémová integrace procesů ve zdravotnictví Autor diplomové práce: Bc. Jana Švagriková Vedoucí diplomové práce: MUDr. Jan Bruthans, Ph.D. Kladno 2018 PROHLÁŠENÍ Prohlašuji, že jsem diplomovou práci s názvem „Programové vybavení pro provoz nukleární medicíny“ vypracovala samostatně a použila k tomu úplný výčet citací použitých pramenů, které uvádím v seznamu přiloženém k diplomové práci. Nemám závažný důvod proti užití tohoto školního díla ve smyslu § 60 Zákona č. 121/2000 Sb., o právu autorském, o právech souvisejících s právem autorským a o změně některých zákonů (autorský zákon), ve znění pozdějších předpisů. V Kladně 18.05.2018 …...….………...………………... Bc. Jana Švagriková PODĚKOVÁNÍ Na tomto místě bych ráda poděkovala svému vedoucímu diplomové práce panu MUDr. Janu Bruthansovi, Ph.D. za vedení a připomínky k mé práci, jeho rady a konzultace. Dále bych chtěla poděkovat všem osloveným společnostem za jejich informace a poskytnuté materiály. Poděkování patří také všem zúčastněným odborníkům. V neposlední řadě děkuji své rodině a přátelům. ABSTRAKT Diplomová práce s názvem „Programové vybavení pro provoz nukleární medicíny“ se zaměřuje na zhodnocení existujících softwarových produktů, zejména radiologických informačních systémů pro využití v provozu nukleární medicíny. Hlavním cílem bylo zmapování současného aktuálního stavu problematiky s důrazem na Českou republiku. Vybrané systémy byly nejprve popsány pomocí deskriptivní metody. Pomocí analýzy SWOT byly zhodnoceny možnosti jednotlivých systémů. Pro výběr ideální varianty systémů byla zvolena metoda multikriteriálního rozhodování, metoda TOPSIS. Závěrem byl vytvořen návrh na doporučení pro uživatele systémů a celkové zhodnocení formou diskuse. -

High-Confidence Medical Devices: Cyber-Physical Systems for 21St Century Health Care
The NITRD Program The Networking and Information Technology Research and Development (NITRD) Program, one of the few formal interagency R&D activities within the Federal government, comprises the Government’s main unclassified R&D investments in advanced networking, computing, software, and related information technology (IT). The NITRD Program also supports research in the socioeconomic implications of IT and in development of a highly skilled IT workforce. Now in its 18th year, NITRD provides a framework and mechanisms for active coordination among 13 Federal research agencies; many other agencies with IT interests also participate in NITRD activities. NITRD is authorized by Congress through the High-Performance Computing Act of 1991 (Public Law 102-194), the Next Generation Internet Research Act of 1998 (Public Law 105-305), and the America COMPETES Act of 2007 (Public Law 110-69). The NITRD agencies work together in eight major research areas – called Program Component Areas (PCAs). In each PCA, agency program managers participate in an Interagency Working Group (IWG) or Coordinating Group (CG) that coordinates R&D activities and preparation of the annual Supplement to the President’s Budget for the NITRD Program. The PCAs are: High End Computing Infrastructure and Applications (HEC I&A), High End Computing Research and Development (HEC R&D), Cyber Security and Information Assurance (CSIA), Human Computer Interaction and Information Management (HCI&IM), Large Scale Networking (LSN), High Confidence Software and Systems (HCSS), Social, Economic, and Workforce Implications of IT and IT Workforce Development (SEW), and Software Design and Productivity (SDP). High Confidence Software and Systems R&D NITRD’s HCSS PCA supports R&D in scientific foundations and innovative and enabling software and hardware technologies for the design, control, assurance, verification and validation, and certification of complex, networked, distributed computing systems and cyber-physical (IT-enabled) systems such as aircraft and power grids. -

MDCG 2019-11 Guidance on Qualification and Classification of Software in Regulation (EU) 2017/745 – MDR and Regulation (EU) 2017/746 – IVDR
Medical Device Medical Device Coordination Group Document MDCG 2019-11 MDCG 2019-11 Guidance on Qualification and Classification of Software in Regulation (EU) 2017/745 – MDR and Regulation (EU) 2017/746 – IVDR October 2019 This document has been endorsed by the Medical Device Coordination Group (MDCG) established by Article 103 of Regulation (EU) 2017/745. The MDCG is composed of representatives of all Member States and it is chaired by a representative of the European Commission. The document is not a European Commission document and it cannot be regarded as reflecting the official position of the European Commission. Any views expressed in this document are not legally binding and only the Court of Justice of the European Union can give binding interpretations of Union law. Guidance on Qualification and Classification of Software October 2019 Guidance on Qualification and Classification of Software in Regulation (EU) 2017/745 – MDR and Regulation (EU) 2017/746 – IVDR Page 1 of 28 Table of Contents 1. Scope and purpose of this document 3 2. Definitions and abbreviations 3 3. Qualification 6 3.1. Introduction to qualification criteria 6 3.2. Medical Device Software (MDSW) 7 3.3. ‘Software driving or influencing the use of a medical device’ 8 3.4. Qualification criteria of MDSW as an in vitro diagnostic medical device 10 4. Classification of MDSW per MDR 2017/745 12 4.1. Implementing Rules 12 4.2. Classification Rules 12 5. Classification and implementing rules per IVDR 2017/746 15 5.1. Implementing Rules: 15 5.2. Classification Rules: 15 6. Considerations on placing on the market and conformity assessment of MDSW 16 6.1. -

Software & Services
SOFTWARE & SERVICES Recognizing the software and service organizations that excelled in helping providers deliver better patient care PHYSICIAN PRACTICE SOLUTIONS BEST IN KLAS AMBULATORY EMR (1–10 PHYSICIANS) HOW DO VENDOR SOLUTIONS COMPARE? WHO IS KONFIDENCE SCORE TREND LEVEL 1. Cerner PowerChart Ambulatory BEST IN KLAS? 84.3 +13% üü 2. Amazing Charts 83.3 +4% üüü 3. SRSsoft EHR 81.0 -11% üü 4. athenahealth athenaClinicals 80.9 -7% üüü 5. Greenway PrimeSUITE Chart 79.1 -2% üüü HOW DO THEY SCORE? 6. Aprima EHR IN FIVE KEY PERFORMANCE CATEGORIES 78.2 -1% üüü 100 92.4 7. ADP AdvancedMD EHR +3% üüü 90 84.9 78.0 80.6 81.7 81.2 80 8. e-MDs Chart -13% üüü 70 74.0 60 AVG. SEGMENT MKT. 9. GE Healthcare Centricity Practice Solution EMR +1% üü PHYSICIAN PRACTICE PHYSICIAN 50 73.8 40 T10. eClinicalWorks EHR 73.4 -7% üüü 30 20 T10. Henry Schein MicroMD EMR 73.4 +4% üü 10 12. NextGen Healthcare EHR 0 65.1 -3% üüü SALES & FUNCTIONALITY GENERAL CONTRACTING & UPGRADES 13. Allscripts Professional EHR 64.7 -6% üüü SERVICE & IMPLEMENTATION SUPPORT 14. McKesson Practice Partner -14% & TRAINING 48.0 üü 0 10 20 30 40 50 60 70 80 90 100 SEE HOW OTHER VENDOR SOLUTIONS SCORE AT KLASRESEARCH.COM HOW DO THE TOP THREE SOLUTIONS TREND? SOLUTIONS NOT RANKED PRELIMINARY DATA PRODUCTS CompuGroup Medical Enterprise EHR (HEHR)*..................71.0 MIE WebChart EMR* ............................................................75.7 MTBC EMR* .........................................................................82.1 Optum Physician EMR* ........................................................79.6 -

Experimentální Porovnání Knihoven Na Detekci Emocí Pomocí Webkamery
MASARYKOVA UNIVERZITA FAKULTA INFORMATIKY Û¡¢£¤¥¦§¨ª«¬Æ°±²³´µ·¸¹º»¼½¾¿Ý Experimentální porovnání knihoven na detekci emocí pomocí webkamery DIPLOMOVÁ PRÁCE Bc. Milan Záleský Brno, podzim 2015 Prohlášení Prohlašuji, že tato diplomová práce je mým p ˚uvodnímautorským dílem, které jsem vypracoval samostatnˇe.Všechny zdroje, prameny a literaturu, které jsem pˇrivypracování používal nebo z nich ˇcerpal,v práci ˇrádnˇecituji s uvedením úplného odkazu na pˇríslušnýzdroj. Bc. Milan Záleský Vedoucí práce: RNDr. Zdenek Eichler i Klíˇcováslova OpenCV, facial expression analysis, webcam, experiment, emotion, detekce emocí, experimentální porovnání ii Podˇekování DˇekujiRNDr. Zdenku Eichlerovi, vedoucímu mé práce, za výteˇcnémento- rování, ochotu pomoci i optimistický pˇrístup, který v pr ˚ubˇehumého psaní projevil. Dˇekujisvým pˇrátel˚uma rodinˇeza opakované vyjádˇrenípodpory. V neposlední ˇradˇetaké dˇekujivšem úˇcastník˚umexperimentu, bez jejichž pˇrispˇeníbych porovnání nemohl uskuteˇcnit. iii Obsah 1 Úvod ...................................1 2 Rozpoznávání emocí ..........................2 2.1 Co je to emoce? . .2 2.2 Posouzení emocí . .3 2.3 Využití informaˇcníchtechnologií k rozpoznávání emocí . .3 2.3.1 Facial Action Coding System . .5 2.3.2 Computer vision . .6 2.3.3 OpenCV . 11 2.3.4 FaceTales . 13 3 Metoda výbˇeruknihoven pro rozpoznávání emocí ........ 15 3.1 Kritéria užšího výbˇeru . 15 3.2 Instalace knihoven . 17 3.3 Charakteristika knihoven zvolených pro srovnání . 20 3.3.1 CLMtrackr . 20 3.3.2 EmotionRecognition . 22 3.3.3 InSight SDK . 24 3.3.4 Vzájemné porovnání aplikací urˇcenýchpro experiment 25 4 Návrh experimentu ........................... 27 4.1 Pˇrípravaexperimentálního porovnání knihoven . 28 4.2 Promˇennéexperimentu . 29 4.2.1 Nezávislé promˇenné. 29 4.2.2 Závislé promˇenné . 29 4.2.3 Sledované promˇenné . 30 4.2.4 Vnˇejšípromˇenné . -
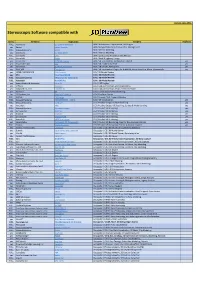
Stereo Software List
Version: June 2021 Stereoscopic Software compatible with Stereo Company Application Category Duplicate FULL Xeometric ELITECAD Architecture BIM / Architecture, Construction, CAD Engine yes Bexcel Bexcel Manager BIM / Design, Data, Project & Facilities Management FULL Dassault Systems 3DVIA BIM / Interior Modeling yes Xeometric ELITECAD Styler BIM / Interior Modeling FULL SierraSoft Land BIM / Land Survey Restitution and Analysis FULL SierraSoft Roads BIM / Road & Highway Design yes Xeometric ELITECAD Lumion BIM / VR Visualization, Architecture Models yes yes Fraunhofer IAO Vrfx BIM / VR enabled, for Revit yes yes Xeometric ELITECAD ViewerPRO BIM / VR Viewer, Free Option yes yes ENSCAPE Enscape 2.8 BIM / VR Visualization Plug-In for ArchiCAD, Revit, SketchUp, Rhino, Vectorworks yes yes OPEN CASCADE CAD CAD Assistant CAx / 3D Model Review yes PTC Creo View MCAD CAx / 3D Model Review FULL Dassault Systems eDrawings for Solidworks CAx / 3D Model Review FULL Autodesk NavisWorks CAx / 3D Model Review yes Robert McNeel & Associates. Rhino (5) CAx / CAD Engine yes Softvise Cadmium CAx / CAD, Architecture, BIM Visualization yes Gstarsoft Co., Ltd HaoChen 3D CAx / CAD, Architecture, HVAC, Electric & Power yes Siemens NX CAx / Construction & Manufacturing Yes 3D Systems, Inc. Geomagic Freeform CAx / Freeform Design FULL AVEVA E3D Design CAx / Process Plant, Power & Marine FULL Dassault Systems 3DEXPERIENCE - CATIA CAx / VR Visualization yes FULL Dassault Systems ICEM Surf CGI / Product Design, Surface Modeling yes yes Autodesk Alias CGI / Product -

Electronic Medical Records Software January 2019
ELECTRONIC MEDICAL RECORDS SOFTWARE JANUARY 2019 Powered by Methodology CONTENTS 3 Introduction 5 Defining Electronic Medical Records Software 6 FrontRunners (Small Vendors) 8 FrontRunners (Enterprise Vendors) 10 Runners Up 16 Methodology Basics 2 INTRODUCTION his FrontRunners analysis Enterprise Vendor graphic had a Tis a data-driven assessment minimum qualifying score of 3.51 identifying products in the for Usability and 3.50 for User Electronic Medical Records Recommended, while the Small software market that offer the Vendor graphic had a minimum best capability and value for small qualifying score of 3.21 for Usability businesses. For a given market, and 3.25 for User Recommended. products are evaluated and given a score for Usability (x-axis) and To be considered for the Electronic User Recommended (y-axis). Medical Records FrontRunners, a FrontRunners then plots 10-15 product needed a minimum of 20 products each on a Small Vendor user reviews published within 18 and an Enterprise Vendor graphic, months of the evaluation period. based on vendor business size, per Products needed a minimum user category. rating score of 3.0 for both Usability and User Recommended in both the In the Electronic Medical Records Small and Enterprise graphics. FrontRunners infographic, the 3 INTRODUCTION The minimum score cutoff to be included in the FrontRunners graphic varies by category, depending on the range of scores in each category. No product with a score less than 3.0 in either dimension is included in any FrontRunners graphic. For products included, the Usability and User Recommended scores determine their positions on the FrontRunners graphic. -

Ehr Software Vendor Directory
EHR SOFTWARE VENDOR DIRECTORY C M CONVERTED MEDIA Software Features Appointment Management Billing Management Clinical Workflow 1ST PROVIDERS CHOICE EHR Document Management Insurance and Claims 1st Providers Choice EHR is a leader in specialty specific ambulatory EHR and practice management software with Lab Integration over 30 specialty-centric customizable templates. Medical Templates Patient Demographics Designed for both small and large medical practices, the EHR manages the entire patient workflow. With 1st Providers Patient Portal Choice patients receive an automatic phone, email and text reminders of appointments while the system also provides Reporting and Analytics automated intake forms and online insurance verification. Scheduling Voice Recognition The 1st Providers Choice suite is fully HIPAA compliant and covers both inpatient and outpatient workflows. A patient e-Prescription portal allows external users to manage appointments and payments while healthcare professionals get access to progress notes and are able to update care plans and other documentation dynamically. Training is provided on site by 1st Providers Choice with online documentation, live chat, and webinars available for existing users. VIEW SOFTWARE REVIEWS, The suite runs on desktops as well as mobile devices, including Android and iOS mobile devices. 1st Providers Choice SCREENSHOTS & MORE is an entirely web-based EHR system. VIEW COMPLETE PROFILE Software Features Appointment Management Billing Management Clinical Workflow Document Management EM Coding ADVANCEDEHR Insurance and Claims AdvancedEHR provides functionality for medical practices as well as components designed for administrative and Lab Integration billing use. Medical Templates Patient Demographics For medical practices, AdvancedEHR provides a patient portal for admissions and check-ins alongside document Patient History management, e-prescriptions and more. -

The Demand for Medical Billing
Welcome to Copyright © 1996 – 2010 by ClaimTek Systems. Tel: (800) -224 - 7450 Copyright © 1996 – 2010 by ClaimTek Systems 1 All rights reserved. No part of this manual may be reproduced in any form or by any means, including photocopying, scanning, or printing without the permission of ClaimTek Systems. For any questions, please contact ClaimTek at: ClaimTek Systems 3943 Irvine Blvd., #39 Irvine, CA 92602-2400 Tel: (800) 224-7450 2 Tel: (800) -224 - 7450 Copyright © 1996 – 2010 by ClaimTek Systems Table of Contents Medical Billing & Electronic Claims Processing Introduction 5 The Demand for Electronic Claims Processing 7 A Fast Growing Industry 9 What the Media Says 10 The Background: Manual Billing 11 The Background: Electronic Billing 12 Manual Vs. Electronic Billing 13 Reasons Why Most Healthcare Providers Do Not File Electronically 14 Four Reasons Why Doctors Need to File Electronically 14 Prospects for Your Services 15 Services You Can Offer 16 What Income Potential Can I Expect? 19 How to Create a Business Plan 22 Software – MedOffice® Practice Management 23 Software – MedOffice® Practice Management Remote 25 Three Additional Software Programs 26 Additional Software / Services You Can Offer 27 Comprehensive Marketing Support 32 How Do We Train You? 40 Our Programs and Packages 45 Why Choose ClaimTek? 47 Frequently Asked Questions 49 Summary: Features of MedOffice® Software 53 Programs & Prices: PREFERRED, PRINCIPAL & ESSENTIAL Programs 54-58 Tel: (800) -224 - 7450 Copyright © 1996 – 2010 by ClaimTek Systems 3 4 Tel: (800) -224 - 7450 Copyright © 1996 – 2010 by ClaimTek Systems Introduction Welcome To ClaimTek’s Medical Billing Business Opportunity Dear Entrepreneur, I am proud and honored to be able to offer you the most comprehensive and professional Medical Billing System you can find anywhere. -

An Optokinetic Nystagmus Detection Method for Use with Young Children
Medical Devices and Systems Received 4 December 2014; revised 16 February 2015; accepted 24 February 2015. Date of publication 5 March 2015; date of current version 24 March 2015. Digital Object Identifier 10.1109/JTEHM.2015.2410286 An Optokinetic Nystagmus Detection Method for Use With Young Children MEHRDAD SANGI1, (Student Member, IEEE), BENJAMIN THOMPSON2, AND JASON TURUWHENUA1 1Auckland Bioengineering Institute, University of Auckland, Auckland 1010, New Zealand 2Department of Optometry and Vision Science, University of Auckland, Auckland 1010, New Zealand CORRESPONDING AUTHOR: J. TURUWHENUA ([email protected]) This work was supported in part by the Auckland Bioengineering Institute and in part by the University of Auckland Faculty Research Development Fund under Grant 1165695. This paper has supplementary downloadable material available at http://ieeexplore.ieee.org., provided by the author. ABSTRACT The detection of vision problems in early childhood can prevent neurodevelopmental disorders such as amblyopia. However, accurate clinical assessment of visual function in young children is challenging. optokinetic nystagmus (OKN) is a reflexive sawtooth motion of the eye that occurs in response to drifting stimuli, that may allow for objective measurement of visual function in young children if appropriate child- friendly eye tracking techniques are available. In this paper, we present offline tools to detect the presence and direction of the optokinetic reflex in children using consumer grade video equipment. Our methods are tested on video footage of children (N D 5 children and 20 trials) taken as they freely observed visual stimuli that induced horizontal OKN. Using results from an experienced observer as a baseline, we found the sensitivity and specificity of our OKN detection method to be 89.13% and 98.54%, respectively, across all trials. -

Rt Visage Download
Rt visage download click here to download Visage is a contemporary blending of stunning, refined visuals, combined with a to Visage template downloads, updates, and the Visage support forum. Visage, the July theme release, is a contemporary blending of stunning, refined visuals, combined with a polished and powerful undertone, providing a. Uncertainty is the root of all fear Wander through the halls, explore each room and every corner of the house in search of an escape route. Uncertainty will. FaceTrack includes face tracking, head tracking, face detection and gaze tracking. RT Visage v - Wordpress Theme Demo: http:www.doorway.ru Turbobit download: http:www.doorway.ru Rapidgator download. SadSquare Studio is raising funds for Visage (Psychological horror game) Going this route doesn't exclude the possibility of us taking on a. GPS Industries Visage Golf Downloads Missing: rt. Visage 7 offers immediate differentiation for imaging organizations seeking to Download Visage 7 Rays Case Study – Acrobat pdf k. pdf Missing: rt. Download Visage Lab for PC - Use Andy OS to run any mobile app Support: we maintain an online real-time Facebook support group if you. Real-time telemetry information was displayed by VisAGE as it was downloaded GenSAA/Genie provided the ability to download telemetry data, maintain. Real time telemetry information was displayed by VisAGE as it was GenSAA/Genie was used to download telemetry data and maintain scheduling information. RT Visage v for Joomla , Visage, the December template have to click to download the www.doorway.ru, in germany it is possible. i use. Visage Mobile Golf Information System: revenue-improvement technology for golf operations.