The Role of Central Mechanisms in the Anti-Inflammatory Effect of Amitriptyline on Carrageenan-Induced Paw Edema in Rats
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Product List March 2019 - Page 1 of 53
Wessex has been sourcing and supplying active substances to medicine manufacturers since its incorporation in 1994. We supply from known, trusted partners working to full cGMP and with full regulatory support. Please contact us for details of the following products. Product CAS No. ( R)-2-Methyl-CBS-oxazaborolidine 112022-83-0 (-) (1R) Menthyl Chloroformate 14602-86-9 (+)-Sotalol Hydrochloride 959-24-0 (2R)-2-[(4-Ethyl-2, 3-dioxopiperazinyl) carbonylamino]-2-phenylacetic 63422-71-9 acid (2R)-2-[(4-Ethyl-2-3-dioxopiperazinyl) carbonylamino]-2-(4- 62893-24-7 hydroxyphenyl) acetic acid (r)-(+)-α-Lipoic Acid 1200-22-2 (S)-1-(2-Chloroacetyl) pyrrolidine-2-carbonitrile 207557-35-5 1,1'-Carbonyl diimidazole 530-62-1 1,3-Cyclohexanedione 504-02-9 1-[2-amino-1-(4-methoxyphenyl) ethyl] cyclohexanol acetate 839705-03-2 1-[2-Amino-1-(4-methoxyphenyl) ethyl] cyclohexanol Hydrochloride 130198-05-9 1-[Cyano-(4-methoxyphenyl) methyl] cyclohexanol 93413-76-4 1-Chloroethyl-4-nitrophenyl carbonate 101623-69-2 2-(2-Aminothiazol-4-yl) acetic acid Hydrochloride 66659-20-9 2-(4-Nitrophenyl)ethanamine Hydrochloride 29968-78-3 2,4 Dichlorobenzyl Alcohol (2,4 DCBA) 1777-82-8 2,6-Dichlorophenol 87-65-0 2.6 Diamino Pyridine 136-40-3 2-Aminoheptane Sulfate 6411-75-2 2-Ethylhexanoyl Chloride 760-67-8 2-Ethylhexyl Chloroformate 24468-13-1 2-Isopropyl-4-(N-methylaminomethyl) thiazole Hydrochloride 908591-25-3 4,4,4-Trifluoro-1-(4-methylphenyl)-1,3-butane dione 720-94-5 4,5,6,7-Tetrahydrothieno[3,2,c] pyridine Hydrochloride 28783-41-7 4-Chloro-N-methyl-piperidine 5570-77-4 -

Slang Terms and Code Words: a Reference for Law Enforcement
UNCLASSIFIED Slang Terms and Code Words: A Reference for Law DEA Enforcement Personnel Intelligence DEA-HOU-DIR-022-18 July 2018 ReportBrief 1 UNCLASSIFIED UNCLASSIFIED DEA Intelligence Report Executive Summary This Drug Enforcement Administration (DEA) Intelligence Report contains new and updated information on slang terms and code words from a variety of law enforcement and open sources, and serves as an updated version to the product entitled “Drug Slang Code Words” published by the DEA in May 2017. It is designed as a ready reference for law enforcement personnel who are confronted with hundreds of slang terms and code words used to identify a wide variety of controlled substances, designer drugs, synthetic compounds, measurements, locations, weapons, and other miscellaneous terms relevant to the drug trade. Although every effort was made to ensure the accuracy and completeness of the information presented, due to the dynamics of the ever-changing drug scene, subsequent additions, deletions, and corrections are inevitable. Future addendums and updates to this report will attempt to capture changed terminology to the furthest extent possible. This compendium of slang terms and code words is alphabetically ordered, with new additions presented in italic text, and identifies drugs and drug categories in English and foreign language derivations. Drug Slang Terms and Code Wordsa Acetaminophen and Oxycodone Combination (Percocet®) 512s; Bananas; Blue; Blue Dynamite; Blueberries; Buttons; Ercs; Greenies; Hillbilly Heroin; Kickers; M-30s; -

Examination of Antimicrobial Activity of Selected Non-Antibiotic Medicinal Preparations
Acta Poloniae Pharmaceutica ñ Drug Research, Vol. 69 No. 6 pp.1368ñ1371, 2012 ISSN 0001-6837 Polish Pharmaceutical Society EXAMINATION OF ANTIMICROBIAL ACTIVITY OF SELECTED NON-ANTIBIOTIC MEDICINAL PREPARATIONS HANNA KRUSZEWSKA1*, TOMASZ ZAR BA1 and STEFAN TYSKI1,2 1National Medicines Institute, Department of Antibiotics and Microbiology, 30-34 Che≥mska St., 00-725 Warszawa, Poland 2 Medical University of Warsaw, Department of Pharmaceutical Microbiology, 3 Oczki St., 02-007 Warszawa, Poland Abstract: The aim of this study was to detect and characterize the antimicrobial activity of non-antibiotic drugs, selected from the pharmaceutical products analyzed during the state control performed in National Medicines Institute, Warszawa, Poland. In 2010, over 90 pharmaceutical preparations have been randomly chosen from different groups of drugs. The surveillance study was performed on standard ATCC microbial strains used for drug control: S. aureus, E. coli, P. aeruginosa and C. albicans. It was shown that the drugs listed below inhib- ited growth of at least one of the examined strains: Arketis 20 mg tab. (paroxetine), Buvasodil 150 mg tab. (buflomedile), Halidor 100 mg tab. (bencyclane), Hydroxyzinum espefa 25 mg tab. (hydroxyzine), Norifaz 35 mg tab. (risedronate), Strattera 60 mg cap. (atomoxetine), Tamiflu 75 mg tab. (oseltamivir), Valpro-ratiopharm Chrono 300 mg tab. with longer dissolution (valproate), Vetminth oral paste 24 g+3 g/100 mL (niclozamide, oxybendazol). Strattera cap. showed broad activity spectrum. It inhibited growth of -

Toxicology Cases: 10 Must-Have Drugs to Keep on Hand
Toxicology Cases: 10 Must-Have Drugs To Keep On Hand Acepromazine Atipamezole Cholestyramine Cyproheptadine Diazepam/ Midazolam Used in animals as a Commonly used to Useful for several Should be an additional For controlling mild sedative; much higher reverse dexmedetomidine toxins that undergo treatment for overdoses agitation or seizures from doses than normal may and xylazine. enterohepatic of serotonergic a variety of toxins. be needed when dealing Note: Can reverse recirculation: drugs such as SSRIs, Note: Should not be with amphetamine signs from other cholecalciferol (vitamin amphetamines, tramadol, used for overdoses of toxicosis. alpha agonists such as D3), sago palm, 5-HTP and marijuana amphetamines as it can clonidine, guanfacine, actually worsen clinical Note: Can also be used to cyanobacteria (blue when signs persist despite tizanidine, imidazoline signs. Also contraindicated help control hypertension green algae), digitoxin treatment with first-line and oxymetazoline for seizures caused by caused by stimulants when and some NSAIDs. drugs. decongestants; eye drops, muscimol (isoxazole) nitroprusside is not available and tick collars that contain Note: Some human Note: Only administer while mushroom ingestions. – start with small doses amitraz. and titrate to affect. Always formulations contain xylitol signs of serotonin syndrome monitor blood pressure. and those must not be used are present – most toxicity in dogs. cases only require 1-3 doses. Maropitant Methocarbamol N-Acetylcysteine Naloxone Propranolol Very effective at both For patients presenting Commonly used for Along with reversing Primarily used to treat stopping and preventing with tremors instead acetaminophen toxicosis; opioid exposure, can be sinus tachycardia vomiting. of seizures. Though can be given along used to reverse severe from chocolate, Note: Administration in expensive, there is with SAMe for other CNS depression from amphetamines, SSRIs, recumbent or neurologic nothing that will control hepatotoxins. -

Companion Animal Euthanasia Training Academy
Companion Animal Euthanasia Training Academy Oral and Injectable Pre-Euthanasia Sedatives/Anesthetics for Dogs and Cats Compiled in 2017 by Dr. Kathleen Cooney, U.S. CAETA instructor Disclaimer: These protocols are meant to provide options for sedation or anesthesia, ranging from mild to deep planes of sleep. Intraorgan injections are only permissible during periods of heavy sedation or anesthesia wherein the animal is pain-free and unaware the injection is occurring. When in doubt, CAETA recommends more drug be given to ensure a pain-free death. Giving sedative or anesthetic drugs to frail and compromised animals can result in adverse reactions or death itself. Drug and drug combinations can be administered intramuscular(IM) or subcutaneous (SQ) unless otherwise specified. Oral drugs may not elicite the desired depth of sleep every time. Many factors affect rate of absorption and uptake. Higher doses may be given initially or when it becomes clear the pet requires more. Dr. Cooney’s Injectable Protocols (IM and SQ) Dogs Sedation protocol (SQ preferred) • Dexmedetomidine (0.5mg/ml) = 0.1 ml per 10# minus 0.1ml (can decrease total by 0.2mls over 50# bw) [can substitute with Xylazine (100mg/ml) = 0.1ml per 10#] • Nalbuphine (20mg/ml) = 0.1 ml per 10# or Butorphanol (10mg/ml) = 0.1 ml per 10# • Acepromazine (10mg/ml) = 0.05 ml per 10# Anesthesia protocol • Telazol® (100mg/ml) = 0.1 ml per 10# • Acepromazine (10mg/ml) = 0.1 ml per 10# • Xylazine (100mg/ml) = 0.025 ml per 10# Cats Anesthesia protocol • Telazol®* (100mg/ml) = 0.15 ml for 2-5# 0.25 ml for 5-10# 0.3 ml for 10+# • Acepromazine (10mg/ml) = 0.1 ml for all BW Anesthesia protocol • Alfaxalone (10mg/ml) = 1.1 -1.5ml for most cats • Nalbuphine (20mg/ml) = 0.2ml for all BW • Acepromazine (10mg/ml)=0.1ml for all BW BW = body weight # = pounds mg = milligram ml = milliliter Various Other Injectable Protocols Shared by Veterinary Colleagues, compiled in 2016 Dogs 1. -

Kentucky Horse Racing Commission Withdrawal Guidelines Thoroughbred; Standardbred; Quarter Horse, Appaloosa, and Arabian KHRC 8-020-2 (11/2018)
Kentucky Horse Racing Commission Withdrawal Guidelines Thoroughbred; Standardbred; Quarter Horse, Appaloosa, and Arabian KHRC 8-020-2 (11/2018) General Notice Unless otherwise specified in these withdrawal guidelines or the applicable regulations and statutes, the following withdrawal guidelines are voluntary and advisory. The guidelines are recommendations based on current scientific knowledge that may change over time. A licensee may present evidence of full compliance with these guidelines to the Kentucky Horse Racing Commission (the “Commission” or “KHRC”) and the stewards as a mitigating factor to be used in determining violations and penalties. These withdrawal interval guidelines assume that administration of medications will be performed at doses that are not greater than the manufacturer’s maximum recommended dosage. Medications administered at dosages above manufacturer’s recommendations, in compounded formulations and/or in combination with other medications and/or administration inside the withdrawal interval may result in test sample concentrations above threshold concentrations that could lead to positive test results and the imposition of penalties. The time of administration of an orally administered substance, for the purposes of withdrawal interval, shall be considered to be the time of complete ingestion of the medication by the horse via eating or drinking. Brand names of medications, where applicable, are listed in parentheses or brackets following the generic name of a drug. In addition to the requirements contained in KRS Chapter 13A, the KHRC shall give notice of an amendment or addition to these withdrawal guidelines by posting the change on the KHRC website and at all Kentucky racetracks at least two weeks before the amendment or addition takes legal effect. -

Adrenoceptors Regulating Cholinergic Activity in the Guinea-Pig Ileum 1978) G.M
- + ! ,' Br. J. Pharmac. (1978), 64, 293-300. F'(O t.,," e reab- ,ellular PHARMACOLOGICAL CHARACTERIZATION OF THE PRESYNAPTIC _-ADRENOCEPTORS REGULATING CHOLINERGIC ACTIVITY IN THE GUINEA-PIG ILEUM 1978) G.M. Departmentof Pharmacology,Allen and HzmburysResearchLimited, Ware, Hertfordshire,SG12 ODJ I The presynaptic ct-adrenoceptors located on the terminals of the cholinergic nerves of the guinea- pig myenteric plexus have been characterized according to their sensitivities to at-adrenoceptor agonists and antagonists. 2 Electrical stimulation of the cholinergic nerves supplying the longitudinal muscle of the guinea-pig ! ileum caused a twitch response. Clonidine caused a concentration-dependent inhibition of the twitch i response; the maximum inhibition obtained was 80 to 95_o of the twitch response. Oxymetazoline and xylazine were qualitatively similar to clonidine but were about 5 times less potent. Phenylephrine and methoxamine also inhibited the twitch response but were at least 10,000 times less potent than clonidine. 3 The twitch-inhibitory effects of clonidine, oxymetazoline and xylazine, but not those of phenyl- ephrine or methoxamine, were reversed by piperoxan (0.3 to 1.0 lag/ml). 4 Lysergic acid diethylamide (LSD) inhibited the twitch response, but also increased the basal tone of the ileum. Mepyramine prevented the increase in tone but did not affect the inhibitory action of LSD. Piperoxan or phentolamine only partially antagonized the inhibitory effect of LSD. 5 Phentolamine, yohimbine, piperoxan and tolazoline were potent, competitive antagonists of the inhibitory effect of clonidine with pA2 values of 8.51, 7.78, 7.64 and 6.57 respectively. 6 Thymoxamine was a weak antagonist of clonidine; it also antagonized the twitch-inhibitory effect of morphine. -

Meperidine Elisa Kit Instructions Product #131219 & 131215 (Rtu) Forensic Use Only
Neogen Corporation 944 Nandino Blvd., Lexington KY 40511 USA 800/477-8201 USA/Canada | 859/254-1221 Fax: 859/255-5532 | E-mail: [email protected] | Web: www.neogen.com/Toxicology MEPERIDINE ELISA KIT INSTRUCTIONS PRODUCT #131219 & 131215 (RTU) FORENSIC USE ONLY INTENDED USE: For the determination of trace quantities of Meperidine and Normeperidine and/or other metabolites in human urine, blood, or oral fluid. DESCRIPTION Neogen Corporation’s Meperidine ELISA (Enzyme-Linked ImmunoSorbent Assay) test kit is a qualitative one-step kit designed for use as a screening device for the detection of drugs and/or their metabolites. The kit was designed for screening purposes and is intended for forensic use only. It is recommended that all suspect samples be confirmed by a quantitative method such as gas chromatography/mass spectrometry (GC/MS). ASSAY PRINCIPLES Neogen Corporation’s test kit operates on the basis of competition between the drug or its metabolite in the sample and the drug-enzyme conjugate for a limited number of antibody binding sites. First, the sample or control is added to the microplate. Next, the drug-enzyme conjugate is added and the mixture is incubated at room temperature. During this incubation, the drug in the sample or the drug-enzyme conjugate binds to antibody immobilized in the microplate wells. After incubation, the plate is washed to remove any unbound sample or drug-enzyme conjugate. The presence of bound drug-enzyme conjugate is recognized by the addition of K-Blue® Substrate (TMB). After a 30 minute substrate incubation, the reaction is halted with the addition of an acid stop. -

Euthanasia: Humane and Human Considerations Sheilah Robertson Senior Medical Director Lap of Love Veterinary Hospice
7/22/2019 Euthanasia: Humane and Human Considerations Sheilah Robertson Senior Medical Director Lap of Love Veterinary Hospice 1 “a life is a story, and the way a story ends is one of the most important parts" Dr. Mary Gardner Co-founder LOL 2 1 7/22/2019 Euthanasia is not a failure, but we can fail at euthanasia 3 Who are the stakeholders? Why do we euthanize animals? Technical aspects Controversies Emotional impact 4 2 7/22/2019 Human and humane considerations 5 CLINICIAN MEDICAL ANIMAL CARE STAFF ETHICAL FINANCIAL OWNER PUBLIC EMOTIONAL 6 3 7/22/2019 Euthanasia Eu – good Thanos – death Ending the life of an animal in a way that minimizes or eliminates pain and distress 7 How does terminology affect how we feel about things? UF Maddie’s Shelter Program 8 4 7/22/2019 Why? 9 Day Veterinary 1 Skills SEMESTERS 1 2 3 4 5 6 7 8 9 Where is euthanasia? - Lap of Love Veterinary Hospice - 10 5 7/22/2019 ART SCIENCE 11 11 Pre-requisites for suffering SENTIENCE CONSCIOUSNESS Awareness of: Awake Sensations Anesthetized Emotions Unconscious Feelings 12 6 7/22/2019 Unconsciousness nullifies perception Mellor and Diesch 2006 13 COMMENTARY Sara White JAVMA 2012 Prevention of fetal suffering during Ovariohysterectomy of pregnant animals 14 7 7/22/2019 WHERE? AT HOME COMFORT ROOM EXAM ROOM 15 WHERE? 16 8 7/22/2019 WHERE? Rosebud Indian Reservation, South Dakota 17 Rules, Regulations & Guidelines Drug Enforcement Administration State, and State board rules Standards of care AVMA Euthanasia Guidelines 18 9 7/22/2019 AVMA Guidelines for the Euthanasia of -

Premedication with Alpha-2 Agonists – Procedures for Monitoring Anaesthetic
Vet Times The website for the veterinary profession https://www.vettimes.co.uk Premedication with alpha-2 agonists – procedures for monitoring anaesthetic Author : Lisa Angell, Chris Seymour Categories : RVNs Date : January 1, 2015 Lisa Angell RVN, VTS(Anaesthesia), looks at the benefits, dosing requirements, alpha-2 agonist options and best practice around premedicating small animal patients in veterinary anaesthesia Reviewed by Chris Seymour MA, VetMB, DVA, DipECVAA, PGCert(MedEd), FHEA, MRCVS PREMEDICATION administered before general anaesthesia is widely used in veterinary practice and may be achieved by using a single drug or a combination. Beneficial effects Premedication has several beneficial effects, including: Sedation Many patients become stressed in a hospital environment. Using a sedative as part of a premedication protocol reduces anxiety and stress for the patient and anaesthetist, and minimises the restraint necessary to place an intravenous catheter. A patient stressed at induction of anaesthesia has higher concentrations of circulating catecholamines (adrenaline and noradrenaline); this is important because some anaesthetic drugs can sensitise the heart to the effects of catecholamines and make cardiac arrhythmias more likely 1 / 11 to develop. A sedated animal is less stressed and therefore has less catecholamine release. Analgesia Depending on the reason for general anaesthesia, many patients need an analgesic drug in the premed. If an animal is already in pain, or if the procedure is painful, providing analgesia is essential. Moreover, giving analgesics before tissue damage occurs (if possible) may help to reduce peripheral and central sensitisation and, consequently, pain after surgery may be less severe. This is termed “pre-emptive” analgesia. Some analgesic drugs also provide some sedation, although this may not be enough to reduce stress and anxiety in all patients. -
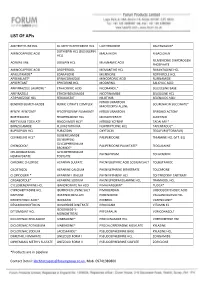
LIST of Apis
LIST OF APIs AMITRIPTYLINE HCL DL-METHYLEPHEDRINE HCL LIOTHYRONINE RALTEGRAVIR* DOTHIEPIN HCL (DOSULEPIN AMINOCAPROIC ACID MALATHION R-BACLOFEN HCL) RILMENIDINE DIHYDROGEN ACRIVASTINE DOXEPIN HCL MEFENAMIC ACID PHOSPHATE AMINOCAPROIC ACID DROPERIDOL MEMANTINE HCL RIMANTADINE HCL APALUTAMIDE* EDARAVONE MILRINONE ROPINIROLE HCL APREMILAST* EFINACONAZOLE MINODRONIC ACID RUFINAMIDE APREPITANT EPHEDRINE HCL MODAFINIL SALICYLIC ACID ARIPIPRAZOLE LAUROXIL* ETHACRYNIC ACID NICORANDIL* SELEGELINE BASE ARIPIRAZOLE ETHOXYBENZAMIDE NICOTINAMIDE SELEGILINE HCL ATIPAMEZOLE HCL FEBUXOSTAT NILOTINIB SELENIUOS ACID NITROFURANTOIN BENDROFLUMETHIAZIDE FERRIC CITRATE COMPLEX SOLIFENACIN SUCCINATE* MACROCRYSTALLINE BENZYL BENZOATE FESOTERODINE FUMARATE NITROFURANTOIN SPIRONOLACTONE BORTEZOMIB FEXOFENADINE HCL MONOHYDRATE SUNITINIB BRETYLIUM TOSYLATE FINGOLIMOD HCL* NITROGLYCERINE TADALAFIL* BRINZOLAMIDE FLUVASTATIN NA NORTRIPTLYINE HCL TAPENTADOL* BUPROPION HCL FURAZIDIN OXYTOCIN TEGAFUR (FTORAFUR) GLIBENCLAMIDE CEVIMELINE HCL* PALIPERIDONE THIAMINE HCL (VIT. B1) (GLYBURIDE) GLYCOPYRRONIUM CHENODIOL* PALIPERIDONE PALMITATE* TIOGUANINE BROMIDE* CHLOROBUTANOL GLYCOPYRRONIUM PHENAZEPAM TOLAZAMIDE HEMIHYDRATE TOSYLATE CHROMIC CHLORIDE HEPARAN SULFATE PHENYLBUTYRIC ACID SODIUM SALT TOLBUTAMIDE CILOSTAZOL HEPARINE CALCIUM PHENYLEPHRINE BITARTRATE TOLCAPONE CLOPIDOGREL* HEPARINE LITHIUM PHENYLEPHRINE HCL TOLTERODINE TARTRATE CRISABOROLE* HEPARINE SODIUM PHENYLPROPANOLAMINE HCL TRAMADOL HCL CYCLOBENZAPRINE HCL IBANDRONATE NA H2O PIMAVANSERIN* TUDCA* CYPROHEPTADINE -
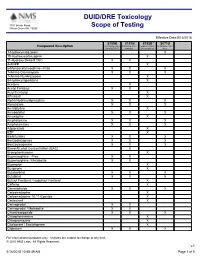
DUID/DRE Toxicology Scope of Testing
DUID/DRE Toxicology 3701 Welsh Road Willow Grove PA, 19090 Scope of Testing Effective Date:5/16/2018 8150B 8151B 8152B* 8071U Compound Description DUID/DRE Panel DUID/DRE Panel Expanded Drug DUID/DRE Panel, ProofPOSITIVE w/alcohol Screen Add-on Urine 1-Hydroxymidazolam X 10-Hydroxycarbazepine X 11-Hydroxy Delta-9 THC X X 4-ANPP X 6-Monoacetylmorphine - Free X X X 7-Amino Clonazepam X X X 7-Amino Flunitrazepam X 9-Hydroxyrisperidone X Acetone X Acetyl Fentanyl X X Acryl Fentanyl X Alfentanil X Alpha-Hydroxyalprazolam X X X Alprazolam X X X Amitriptyline X Amobarbital X X X Amoxapine X Amphetamine X X X Amphetamines X X X Aripiprazole X BZP X Barbiturates X X X Benzodiazepines X X X Benzoylecgonine X X X Blood Alcohol Concentration (BAC) X Brompheniramine X Buprenorphine - Free X X Buprenorphine / Metabolite X X Bupropion X Buspirone X Butabarbital X X X Butalbital X X X Butyryl Fentanyl / Isobutyryl Fentanyl X Caffeine X Cannabinoids X X X Carbamazepine X Carbamazepine-10,11-Epoxide X Carfentanil X Carisoprodol X X Carisoprodol / Metabolite X X Chlordiazepoxide X X X Chlorpheniramine X Chlorpromazine X Citalopram / Escitalopram X Clobazam X X X For informational purposes only. Analytes are subject to change at any time. © 2010 NMS Labs. All Rights Reserved. v.1 5/16/2018 10:58:49 AM Page 1 of 5 8150B 8151B 8152B* 8071U Compound Description DUID/DRE Panel DUID/DRE Panel Expanded Drug DUID/DRE Panel, ProofPOSITIVE w/alcohol Screen Add-on Urine Clomipramine X Clonazepam X X Clonazolam X Clonidine X Clozapine X Cocaethylene X X X Cocaine X