Bioinformatic Analysis Reveals That the Reproductive System Is Potentially at Risk from SARS-Cov-2
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Reproductive Attributes of Polynoid Polychaetes from Hydrothermal Vents on the East Pacific Rise
W&M ScholarWorks Dissertations, Theses, and Masters Projects Theses, Dissertations, & Master Projects 2005 Reproductive Attributes of Polynoid Polychaetes from Hydrothermal Vents on the East Pacific Rise Jessica Lynn Wallace College of William & Mary - Arts & Sciences Follow this and additional works at: https://scholarworks.wm.edu/etd Part of the Marine Biology Commons, and the Oceanography Commons Recommended Citation Wallace, Jessica Lynn, "Reproductive Attributes of Polynoid Polychaetes from Hydrothermal Vents on the East Pacific Rise" (2005). Dissertations, Theses, and Masters Projects. Paper 1539626835. https://dx.doi.org/doi:10.21220/s2-zy51-8j97 This Thesis is brought to you for free and open access by the Theses, Dissertations, & Master Projects at W&M ScholarWorks. It has been accepted for inclusion in Dissertations, Theses, and Masters Projects by an authorized administrator of W&M ScholarWorks. For more information, please contact [email protected]. REPRODUCTIVE ATTRIBUTES OF POLYNOID POLYCHAETES FROM HYDROTHERMAL VENTS ON THE EAST PACIFIC RISE A Thesis Presented to The Faculty of the Department of Biology The College of William and Mary in Virginia In Partial Fulfillment Of the Requirements for the Degree of Master of Science by Jessica Lynn Wallace 2005 APPROVAL SHEET This thesis is submitted in partial fulfillment of the requirements for the degree of Master of Science Jessica L. Wallace Approved by the Committee, August 2005 Dr. Cindy Lee Van Dover, Chair Dr. Paul D. Heideman Dr. Joseph L. Scott To Dad and Ken for inspiring my love of oceanography To Mom and Stephen for their unending love and support TABLE OF CONTENTS Page Acknowledgements v List of Figures vi Abstract vii Introduction 2 Chapter I. -

Human Reproductive Systems Males Vs. Females Learning Goals • Students Will Describe the Basic Anatomy and Physiology of the Male and Female Reproductive Systems
Human Reproductive Systems Males vs. Females Learning Goals • Students will describe the basic anatomy and physiology of the male and female reproductive systems. Gonads are sex organs that create gametes? & excrete sex hormones Gonads are sex organs that create gametes & excrete sex hormones Male gonads are called testes Female gonads are called ovaries -Are the site of sperm production -Are the site of egg production & maturation Gametes are also called sex ?cells, and are used to create offspring with a mixture of genetic information. Gametes are also called sex cells, and are used to create offspring with a mixture of genetic information. Male gametes are called sperm Female gametes are called -produce 300-500 million per 5ml eggs/ova of semen -70,000-100,000 at birth -release 1-2 per month from puberty to menopause. Sex Hormones are chemical? signals that tell the sex organs how to function. Sex Hormones are chemical signals that tell the sex organs how to function. Male hormone is called Female hormones are estrogen testosterone and progesterone -released from the testes -released from the ovary -controls sperm production -controls egg production & release Duct systems help deliver gametes from gonads and are the site of fertilization in females and delivers sperm out of the body in males. Male duct systems include: Epididymis -site of sperm maturation (about 20 days for sperm to mature) Male duct systems include: Vas deferens -Tube for sperm to travel through as they leave the testes Male duct systems include: Urethra -shared tube for release of semen from reproductive tract and urine from the bladder. -

REVIEW Physiological Dependence on Copulation in Parthenogenetic Females Can Reduce the Cost of Sex
ANIMAL BEHAVIOUR, 2004, 67, 811e822 doi:10.1016/j.anbehav.2003.05.014 REVIEW Physiological dependence on copulation in parthenogenetic females can reduce the cost of sex M. NEIMAN Department of Biology, Indiana University, Bloomington (Received 6 December 2002; initial acceptance 10 April 2003; final acceptance 27 May 2003; MS. number: ARV-25) Despite the two-fold reproductive advantage of asexual over sexual reproduction, the majority of eukaryotic species are sexual. Why sex is so widespread is still unknown and remains one of the most important unanswered questions in evolutionary biology. Although there are several hypothesized mechanisms for the maintenance of sex, all require assumptions that may limit their applicability. I suggest that the maintenance of sex may be aided by the detrimental retention of ancestral traits related to sexual reproduction in the asexual descendants of sexual taxa. This reasoning is based on the fact that successful reproduction in many obligately sexual species is dependent upon the behavioural, physical and physiological cues that accompany sperm delivery. More specifically, I suggest that although parthenogenetic (asexual) females have no need for sperm per se, parthenogens descended from sexual ancestors may not be able to reach their full reproductive potential in the absence of the various stimuli provided by copulatory behaviour. This mechanism is novel in assuming no intrinsic advantage to producing genetically variable offspring; rather, sex is maintained simply through phylogenetic constraint. I review and synthesize relevant literature and data showing that access to males and copulation increases reproductive output in both sexual and parthenogenetic females. These findings suggest that the current predominance of sexual reproduction, despite its well-documented drawbacks, could in part be due to the retention of physiological dependence on copulatory stimuli in parthenogenetic females. -

Infectious Diseases: Respiratory & Reproductive Systems
Chapter 10 - Lesson 3 Infectious Diseases: Respiratory & Reproductive Systems Nasal Passages and Sinuses Rhinitis is an inflammation of the mucous membranes of the nasal passages, and sinusitis is the inflamma- tion of sinuses. These conditions can be either acute or chronic. Bacterial infections may occur following an acute viral infection or after exposure to inclimate weather. Conjunctivitis (inflammation of eyelids) of- ten accompanies rhinitis and sinusitis. Affected tissues may become red and swollen and Conjunctivitis is an inflammation of the eyelids. produce a mucoid or mucopurulent nasal discharge. In addition drainage from the nostrils, open-mouth breathing and sneezing are also common signs of a re- spiratory infection. Strangles in horses is caused by a streptococcus bacterium and is an inflammation of the sinuses and nasal passages that may include abscessa- tion of associated lymph nodes. Tonsils Tonsillitis is the inflammation of the tonsils. This con- dition is common in dogs and rare in cats. The bacteri- Canine distemper. al infection causes the tonsils to swell resulting in gag- ging, retching, soft coughing, and expulsion of mucus. pneumonia with congestion, hemorrhage, mucus, Lungs edema, and emphysema of lung tissue and air sacs. Bacteria commonly complicate viral lung infections Pneumonitis is an acute or chronic inflammation of by causing collection of pus in the air sacs. the lung tissue and air passages. Symptoms include a deep cough and difficult breathing. Infectious bovine rhinotracheitis (IBR), equine rhi- nopneumonitis, equine influenza, swine influenza, Viral and bacterial infections of the lungs are con- canine distemper, feline viral rhinotracheitis (FVR), tagious and can produce severe damage resulting in feline calicivirus (FCV), fowl infectious bronchitis, Chapter 10 - Infectious Diseases 225 fowl infectious laryngotracheitis (LT), and avian in- fluenza (AI) are viral diseases of the respiratory sys- tem. -

Mating Systems in the Pleurothallidinae (Orchidaceae): Evolutionary and Systematic Implications
LANKESTERIANA 11(3): 207—221. 2011. MATING SYSTEMS IN THE PLEUROTHALLIDINAE (ORCHIDACEAE): EVOLUTIONARY AND SYSTEMATIC IMPLICATIONS EDUARDO LEITE BORBA*, ARIANE RAQUEL BARBOSA, MARCOS CABRAL DE MELO, SAMUEL LOUREIRO GONTIJO & HENRIQUE ORNELLAS DE OLIVEIRA Departamento de Botânica, Instituto de Ciências Biológicas Universidade Federal de MinasGerais, Belo Horizonte, MG, 31270-901, Brazil * Corresponding author: [email protected] ABSTRACT. We developed a project addressing the determination of the reproductive system through experimental pollinations of species in the major genera representing all major lineages of Pleurothallidinae in order to determine occurrence of self-incompatibility in the subtribe, in which group it has possibly appeared for the first time, and how many times it has evolved. Additionally we surveyed the floral biology of species ofOctomeria , a genus with morphological characters typical of bee-pollinated flowers that was previously regarded as mellitophilous. At the moment, all but one of the species studied in selected large genera of the major lineages (Acianthera, Anathallis, Masdevallia, Octomeria, Specklinia, and Stelis) are self-incompatible. The species studied may possess complete, strong or partial self-incompatibility. We found two different sites where self-incompatibility reactions occur, the stigma and the stylar channel, and both sites were not found in the same genus except for Anathallis. In Anathallis, the two groups that differ morphologically (formerly Pleurothallis subgen. Specklinia sect. Muscosae -

The Seahorse Genome and the Evolution of Its Specialized
OPEN ARTICLE doi:10.1038/nature20595 The seahorse genome and the evolution of its specialized morphology Qiang Lin1*§, Shaohua Fan2†*, Yanhong Zhang1*, Meng Xu3*, Huixian Zhang1,4*, Yulan Yang3*, Alison P. Lee4†, Joost M. Woltering2, Vydianathan Ravi4, Helen M. Gunter2†, Wei Luo1, Zexia Gao5, Zhi Wei Lim4†, Geng Qin1,6, Ralf F. Schneider2, Xin Wang1,6, Peiwen Xiong2, Gang Li1, Kai Wang7, Jiumeng Min3, Chi Zhang3, Ying Qiu8, Jie Bai8, Weiming He3, Chao Bian8, Xinhui Zhang8, Dai Shan3, Hongyue Qu1,6, Ying Sun8, Qiang Gao3, Liangmin Huang1,6, Qiong Shi1,8§, Axel Meyer2§ & Byrappa Venkatesh4,9§ Seahorses have a specialized morphology that includes a toothless tubular mouth, a body covered with bony plates, a male brood pouch, and the absence of caudal and pelvic fins. Here we report the sequencing and de novo assembly of the genome of the tiger tail seahorse, Hippocampus comes. Comparative genomic analysis identifies higher protein and nucleotide evolutionary rates in H. comes compared with other teleost fish genomes. We identified an astacin metalloprotease gene family that has undergone expansion and is highly expressed in the male brood pouch. We also find that the H. comes genome lacks enamel matrix protein-coding proline/glutamine-rich secretory calcium-binding phosphoprotein genes, which might have led to the loss of mineralized teeth. tbx4, a regulator of hindlimb development, is also not found in H. comes genome. Knockout of tbx4 in zebrafish showed a ‘pelvic fin-loss’ phenotype similar to that of seahorses. Members of the teleost family Syngnathidae (seahorses, pipefishes de novo. The H. comes genome assembly is of high quality, as > 99% and seadragons) (Extended Data Fig. -

Polygynandry, Extra-Group Paternity and Multiple-Paternity Litters In
Molecular Ecology (2007) 16, 5294–5306 doi: 10.1111/j.1365-294X.2007.03571.x Polygynandry,Blackwell Publishing Ltd extra-group paternity and multiple-paternity litters in European badger (Meles meles) social groups HANNAH L. DUGDALE,*† DAVID W. MACDONALD,* LISA C. POPE† and TERRY BURKE† *Wildlife Conservation Research Unit, Department of Zoology, University of Oxford, Tubney House, Abingdon Road, Tubney OX13 5QL, UK, †Department of Animal and Plant Sciences, University of Sheffield, Western Bank, Sheffield S10 2TN, UK Abstract The costs and benefits of natal philopatry are central to the formation and maintenance of social groups. Badger groups, thought to form passively according to the resource dispersion hypothesis (RDH), are maintained through natal philopatry and delayed dispersal; however, there is minimal evidence for the functional benefits of such grouping. We assigned parentage to 630 badger cubs from a high-density population in Wytham Woods, Oxford, born between 1988 and 2005. Our methodological approach was different to previous studies; we used 22 microsatellite loci to assign parent pairs, which in combination with sibship inference provided a high parentage assignment rate. We assigned both parents to 331 cubs at ≥ 95% confidence, revealing a polygynandrous mating system with up to five mothers and five fathers within a social group. We estimated that only 27% of adult males and 31% of adult females bred each year, suggesting a cost to group living for both sexes. Any strong motivation or selection to disperse, however, may be reduced because just under half of the paternities were gained by extra-group males, mainly from neighbouring groups, with males displaying a mixture of paternity strategies. -

Researchers Reveal Negative Effects of Tributyltin on the Reproductive System of Seahorses 21 December 2020, by Li Yuan
Researchers reveal negative effects of tributyltin on the reproductive system of seahorses 21 December 2020, by Li Yuan The researchers conducted physiological, histological, and transcriptional analyses, and found that high levels of TBT bioaccumulation occurred in male and female seahorses. "TBT leads to ovarian follicular atresia and apoptosis with the elevation of androgen levels, accompanied by the induction of genes associated with lysosomes and autophagosomes," said Prof. Lin. Effects of tributyltin on gonad and brood pouch development of male pregnant lined seahorse Comparative transcriptional analyses revealed the (Hippocampus erectus) at environmentally relevant likely inhibition of spermatogenesis via the concentrations. Credit: SCSIO suppression of cyclic AMP and androgen synthesis. "The transcriptional profiles show that TBT potentially affects the immune system, Seahorses epitomize the exuberance of evolution. angiogenesis, and embryo nourishment of the They have the unique characteristic of male brood pouch, indicating that it has negative effects pregnancy, which includes the carrying of many on the male reproductive system of seahorses," embryos in a brood pouch that incubates and said Prof. Lin. nourishes the embryos, similar to the mammalian placenta. The study reveals that environmental levels of TBT have the potential to affect the reproductive The male pregnancy of seahorses is unique, but efficiency of seahorses, possibly leading to a their reproductive response to environmental reduction in their populations in coastal disturbances has not been clarified. Tributyltin environments. (TBT) is known to have an endocrine disrupting effect on the reproductive system of coastal marine More information: Lu Tang et al. Effects of organisms. tributyltin on gonad and brood pouch development of male pregnant lined seahorse (Hippocampus Researchers led by Prof. -

Mate Choice | Principles of Biology from Nature Education
contents Principles of Biology 171 Mate Choice Reproduction underlies many animal behaviors. The greater sage grouse (Centrocercus urophasianus). Female sage grouse evaluate males as sexual partners on the basis of the feather ornaments and the males' elaborate displays. Stephen J. Krasemann/Science Source. Topics Covered in this Module Mating as a Risky Behavior Major Objectives of this Module Describe factors associated with specific patterns of mating and life history strategies of specific mating patterns. Describe how genetics contributes to behavioral phenotypes such as mating. Describe the selection factors influencing behaviors like mate choice. page 882 of 989 3 pages left in this module contents Principles of Biology 171 Mate Choice Mating as a Risky Behavior Different species have different mating patterns. Different species have evolved a range of mating behaviors that vary in the number of individuals involved and the length of time over which their relationships last. The most open type of relationship is promiscuity, in which all members of a community can mate with each other. Within a promiscuous species, an animal of either gender may mate with any other male or female. No permanent relationships develop between mates, and offspring cannot be certain of the identity of their fathers. Promiscuous behavior is common in bonobos (Pan paniscus), as well as their close relatives, the chimpanzee (P. troglodytes). Bonobos also engage in sexual activity for activities other than reproduction: to greet other members of the community, to release social tensions, and to resolve conflicts. Test Yourself How might promiscuous behavior provide an evolutionary advantage for males? Submit Some animals demonstrate polygamous relationships, in which a single individual of one gender mates with multiple individuals of the opposite gender. -

Chapter 28 Reproductive System (RS)
Chapter 28 Reproductive System (RS) AP2 Chapter 28 1 Chapter 28 Outline I. Anatomy of the Male RS II. Physiology of the male RS III. Anatomy of the female RS IV. Physiology of the female RS V. FX of aging on the RS AP2 Chapter 28 2 The Reproductive System • Controls the development of the structural & fxnal diff between ♀ and ♂ • Not only are reproductive structures different but integumentary, muscular & skeletal system diff’s also exist • Reproduction: essential to all living organisms (Thus fxnal RS are required to successfully reproduce) • The ♂ RS is designed to prod sperm & deliver it to the ♀ oocyte • The ♀ RS is designed to prod oocytes & receive sperm from the ♂ RS. It is also intimately involved with nurturing the development of the new individual until and well after birth. AP2 Chapter 28 3 I. Anatomy of the ♂ RS AP2 Chapter 28 4 I. Anatomy of the ♂ RS Pathway • Consists of: • Temp sensitive sperm cells 1. Testes develop outside the body 2. Ducts cavity in the testes & ED Epididymides (ED) Ductus Differentia (DD) • DD lead from the testes into Urethra the pelvis & join with the SV 3. Accessory glands to form the ampullae Seminal Vesicles (SV) Prostate Gland (PG) • Extensions called Bulbourethral Gland (BG) ejaculatory ducts pass thru 4. Supporting Structures the PG & empty into the Scrotum urethra w/in the PG Penis • Urethra exits the pelvis & passes thru the penis to the outside of the body 5 I. Anatomy of the ♂ RS: Scrotum • Holds testes in compartments separated by an incomplete CT septum • Raphe: external irregular midline ridge on the scrotum that continues to the anus (posteriorly) and Base of the penis (anteriorly) • Outer layers of scrotum: – Skin Raphe – Superficial Fascia (LCT) – Dartos Muscle • 2 muscles control the scrotum’s hold on the testes: – Dartos muscle & cremaster muscle – Hot they both relax & testes move away from the body – Cold and they contract bringing testes toward the warmth of the body – Normal sperm formation is temperature sensitive 6 I. -

The Human Reproductive System
ANATOMY- PHYSIOLOGY-REPRODUCTIVE SYSTEM - IN RESPONSE TO CONVID 19 APRIL 2, 2020 nd Dear students and parents, April 2 , 2020 Beginning two days prior to our last day at school I issued work packets to all students in all classed; the content of which was spanning a two-three week period. Now that our removal from school will continue to at least May 1st, I have provided the following work packets which will span the remainder of the year, should our crisis continue. The following folders are available: ANATOMY – PHYSIOLOGY 1. Packet – THE HUMAN REPRODUCATIVE AND ENDOCRINE SYSTEMS. 2. Packet- THE HUMAN NERVOUS SYSTEM 3. Packet handed our prior to our last day: THE HUMAN EXCRETORY SYSTEM ZOOLOGY 1. Packet- STUDY OF THE CRUSTACEANS 2. Packet- STUDY OF THE INSECTS 3. Packet- handed our prior to our last day- INTRODUCTION TO THE ARTRHROPODS- CLASSES MYRIAPODA AND ARACHNIDA AP BIOLOGY – as per the newly devised topics of study focus, structure of adapted test, test dates and supports provided as per the guidelines and policies of The College Board TO ALL STUDENTS! THESE PACKETS WILL BE GUIDED BY THE SAME PROCEDURES WE EMBRACED DURING FALL TECH WEEK WHERE YOU ARE RESPONSIBLE FOR THE WORK IN THE PACKETS- DELIVERED UPON YOUR RETURN TO SCHOOL OR AS PER UNFORESEEN CHANGES WHICH COME OUR WAY. COLLABORATION IS ENCOURAGED- SO STAY IN TOUCH AND DIG IN! YOUR PACKETS WILL BE A NOTEBOOK GRADE. EVENTUALLY YOU SHALL TAKE AN INDIVIDUAL TEST OF EACH PACKET = AN EXAM GRADE! SCHOOL IS OFF SITE BUT NOT SHUT DOWN SO PLEASE DO THE BODY OF WORK ASSIGNED IN THE PACKETS PROVIDED. -
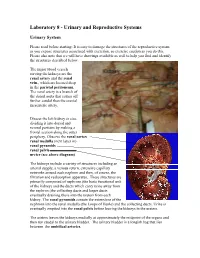
Laboratory 8 - Urinary and Reproductive Systems
Laboratory 8 - Urinary and Reproductive Systems Urinary System Please read before starting: It is easy to damage the structures of the reproductive system as you expose structures associated with excretion, so exercise caution as you do this. Please also note that we will have drawings available as well to help you find and identify the structures described below. The major blood vessels serving the kidneys are the Renal renal artery and the renal pyramid vein., which are located deep in the parietal peritoneum. The renal artery is a branch of the dorsal aorta that comes off Renal further caudal than the cranial pelvis mesenteric artery. Dissect the left kidney in situ, dividing it into dorsal and ventral portions by making a frontal section along the outer periphery. Observe the renal cortex renal medulla (next layer in) renal pyramids renal pelvis ureter (see above diagram) The kidneys include a variety of structures including an arterial supply, a venous return, extensive capillary networks around each nephron and then, of course, the filtration and reabsorption apparatus. These structures are primarily composed of nephrons (the basic functional unit of the kidney) and the ducts which carry urine away from the nephron (the collecting ducts and larger ducts eventually draining these into the ureters from each kidney. The renal pyramids contain the extensions of the nephrons into the renal medulla (the Loops of Henle) and the collecting ducts. Urine is eventually emptied into the renal pelvis before leaving the kidneys in the ureters. The ureters leaves the kidneys medially at approximately the midpoint of the organs and then run caudal to the urinary bladder.