Skin Lipids from Saudi Arabian Birds
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

OSME List V3.4 Passerines-2
The Ornithological Society of the Middle East, the Caucasus and Central Asia (OSME) The OSME Region List of Bird Taxa: Part C, Passerines. Version 3.4 Mar 2017 For taxa that have unproven and probably unlikely presence, see the Hypothetical List. Red font indicates either added information since the previous version or that further documentation is sought. Not all synonyms have been examined. Serial numbers (SN) are merely an administrative conveninence and may change. Please do not cite them as row numbers in any formal correspondence or papers. Key: Compass cardinals (eg N = north, SE = southeast) are used. Rows shaded thus and with yellow text denote summaries of problem taxon groups in which some closely-related taxa may be of indeterminate status or are being studied. Rows shaded thus and with white text contain additional explanatory information on problem taxon groups as and when necessary. A broad dark orange line, as below, indicates the last taxon in a new or suggested species split, or where sspp are best considered separately. The Passerine Reference List (including References for Hypothetical passerines [see Part E] and explanations of Abbreviated References) follows at Part D. Notes↓ & Status abbreviations→ BM=Breeding Migrant, SB/SV=Summer Breeder/Visitor, PM=Passage Migrant, WV=Winter Visitor, RB=Resident Breeder 1. PT=Parent Taxon (used because many records will antedate splits, especially from recent research) – we use the concept of PT with a degree of latitude, roughly equivalent to the formal term sensu lato , ‘in the broad sense’. 2. The term 'report' or ‘reported’ indicates the occurrence is unconfirmed. -

Eastern Afromontane Biodiversity Hotspot
Ecosystem Profile EASTERN AFROMONTANE BIODIVERSITY HOTSPOT FINAL VERSION 24 JANUARY 2012 Prepared by: BirdLife International with the technical support of: Conservation International / Science and Knowledge Division IUCN Global Species Programme – Freshwater Unit IUCN –Eastern Africa Plant Red List Authority Saudi Wildlife Authority Royal Botanic Garden Edinburgh, Centre for Middle Eastern Plants The Cirrus Group UNEP World Conservation Monitoring Centre WWF - Eastern and Southern Africa Regional Programme Office Critical Ecosystem Partnership Fund And support from the International Advisory Committee Neville Ash, UNEP Division of Environmental Policy Implementation; Elisabeth Chadri, MacArthur Foundation; Fabian Haas, International Centre of Insect Physiology and Ecology; Matthew Hall, Royal Botanic Garden Edinburgh, Centre for Middle Eastern Plants; Sam Kanyamibwa, Albertine Rift Conservation Society; Jean-Marc Froment, African Parks Foundation; Kiunga Kareko, WWF, Eastern and Southern Africa Regional Programme Office; Karen Laurenson, Frankfurt Zoological Society; Leo Niskanen, IUCN Eastern & Southern Africa Regional Programme; Andy Plumptre, Wildlife Conservation Society; Sarah Saunders, Royal Society for the Protection of Birds; Lucy Waruingi, African Conservation Centre. Drafted by the ecosystem profiling team: Ian Gordon, Richard Grimmett, Sharif Jbour, Maaike Manten, Ian May, Gill Bunting (BirdLife International) Pierre Carret, Nina Marshall, John Watkin (CEPF) Naamal de Silva, Tesfay Woldemariam, Matt Foster (Conservation International) -

Anambra Waxbill Study
ABUNDANCE, DISTRIBUTION AND GENETIC ASSOCIATION OF ANAMBRA WAXBILL (Estrilda poliopaeria, L) IN NIGERIA. Ebenezer Olubunmi Coker A.P Leventis Ornithological Research Institute, University of Jos, Biological Conservatory, Amurum Forest Reserve, P.O. Box 13404, Laminga, Jos – East LGA, Jos, Plateau State- Nigeria Report to the African Bird Club of Field work on Anambra Waxbill 27/11/ 2014 to 10/10/2015 ABSTRACT Endemic species are in constant danger of extinction due to natural and anthropogenic causes. The Anambra Waxbill Estrilda poliopareia is an endemic bird species with a small population reported from different locations in Anambra, Bayelsa and Delta States of Nigeria. Faced with ecological and evolutionary pressures, there is an increasing need to evaluate the status of the refugia populations. This study aims to evaluate the abundance, distribution and genetic status of Anambra Waxbill in Nigeria. Surveys have so far been carried out at historical and reported sites of Anambra Waxbill from November 27, 2014 to October 10, 2015. Point transects and mist-netting were employed to census birds and vegetation characteristics measured afterwards. A total of 104 individuals were trapped to collect blood and feather samples for molecular analysis. During this survey, 467 individuals of Anambra Waxbill were recorded in the study sites. The abundance of Anambra Waxbill was not influenced by the vegetation parameters measured; however, the occurence of the species was significantly influenced by the grass height, percentage litter cover and number of stumps. The results suggest that forest clearings with increase in grass height and decreased litter cover may provide opportunities for Anambra Waxbill to colonize new habitats. -

Restricted-Range Bird Species Listed by Family
APPENDIX 1: Restricted-range bird species listed by family NCLUDED here are all the landbird species treated Threat codes Ias having restricted ranges, listed with the coun- 0 Unknown tries in which they breed (but omitting countries in 1 Loss or alteration of habitat 2 Hunting, persecution, egg-collecting (subsistence) which all populations originate from introductions), 3 Disturbance (by humans, stock) the Endemic Bird Areas (and Secondary Areas) in 4 Pollution, pesticides, poisoning which they occur, the broad habitat-types which they 5 Introduced species (predators, competitors, herbivores, prefer, their status and (for those which are classified diseases) 6 Trade, egg-collecting (commercial) as threatened) the major threats which affect them. 7 Natural causes (exacerbated by other influences) Some species are of unknown provenance, and these 8 Small range or population are listed on p. 724. Notes Habitat codes * Taxonomy deviates from Sibley and Monroe (1990, F All forest and D Desert 1993); see EBA (or Secondary Area) account for further woodland types R Rocky areas details and references. The relationship of the new genus S Scrub A Agricultural areas Cryptosylvicola (p. 708) within Sylviinae is unconfirmed, V Savanna X Introduced vegetation and so it has been placed at the end of that subfamily. G Grassland Z Unknown X Extinct in that country or in that EBA/SA. W Wetland 1 Antigua and Barbuda, Dominica, Guadeloupe (to France), Martinique (to France), Montserrat (to UK), Netherlands Status Antilles (to Netherlands), Puerto Rico (to USA), St Lucia, IUCN Red List Categories have been used as applied by Virgin Islands (to UK), Virgin Islands (to USA). -

Biological Diversity of the Republic of Yemen
BIOLOGICAL DIVERSITY OF THE REPUBLIC OF YEMEN ' i ' 7 . .' . ... '- . , " . , .... T ...7v , ~ ~ !, , ,,.... I SH & III)l,I I" "l,'N J~~~ I , l '\ PA - r itwA BIOLOGICAL DIVERSITY ASSESSMENT OF THE REPUBLIC OF YEMEN by Daniel Martin Varisco James Perran Ross Anthony Milroy Editor Michael R. W. Rands July 1992 International Council for Bird Preservatiot1 32 Cambridge Road Girton Cambridge CB3 OPJ United Kingdom CONTENTS Page Editorial iii Acronyms used in this report iv Executive summary v INTRODUCTION 1 Project rationale 1 Scope of work 1 Methodology 2 Status of available data 2 Background on Yemen's development context 3 BIOLOGICAL RESOURCES OF YEMEN 5 Ecosystem variety 5 Survey of flora 11 Survey of fauna 20 ENV.LONMENTAL POLICY 28 Institutional responsibilities 28 Legislation regarding flo-a and forestry 29 Legislation regarding wildlife 30 Summary 30 ENVIRONMENTAL EDUCATION AND AWARENESS 31 Programs in government ministries 31 Media 32 NGO development 32 Summary 33 IMPACT OF DEVELOPMENT ON BIOLOGICAL RESOURCES 34 Overall development policy 34 Case study of Wadi Zabid 34 Integrated development policy and the environment 37 Coastal development 38 CONSERVATION PRIORITIES FOR BIODIVERSITY 41 Development policy 41 Institutional development 42 Conservation awareness 43 Critical areas 43 Critical species 44 Crop genetic diversity 45 Sustainable fisheries and coastal management 45 Summary 45 Page RECOMMENDATIONS FOR CONSERVATION OF BIOLOGICAL Resources 47 General reconunendations 47 Recommendations for USAID 54 ANNEXES: 1 Bibliography 58 -
Boundaries of the Palearctic Region Kees (C
The boundaries of the Palearctic region Kees (C. S.) Roselaar ABSTRACT Although the western and eastern boundaries of the Palearctic region are widely agreed, there is still much debate about the southern boundary. In an attempt to establish a definitive southern boundary, the distribution of 1,037 breeding passerine species in the Palearctic, and adjacent parts of the Afrotropical and Oriental regions north of 5°N, were analysed. The WorldMap computer program was used to combine these maps, to reveal species richness.The variations in the position of the southern boundary suggested by previous authorities gives rise to three zones: north of the northernmost boundary, which is unequivocally Palearctic; south of the southernmost boundary, which is unequivocally Afrotropical or Oriental; and a border region in between, which is analysed in detail here to establish an objective southern boundary for the region. n 1973, during initial meetings of the birds, but analyses of other groups of terrestrial editorial team of a planned handbook of animals by subsequent workers, notably Wallace Ibirds in Europe, one of the topics of debate (1876), supported and expanded his scheme. was the title of the forthcoming work. Several Many books and checklists have now been pub- people opted for the title ‘Birds of the Western lished on the birds of the Palearctic. Their Palearctic’ because it was short and geographi- authors are more or less agreed on the western cally clearly defined; others thought that the and eastern borders of the region, which stretch term ‘Palearctic’ was too little-known in birding from Iceland and Morocco in the west, across to circles to attract potential buyers. -
C:\Users\Jennings\1 ABBA\Forms Updated\Forms\Form 2 3-11.Wpd
ABBA Form 2 (3/11) LIST OF THE BREEDING BIRDS OF ARABIA (Please note this list, including the nomenclature and taxonomic order is under review and will be bought in line with that of the Atlas of the Breeding Birds of Arabia which was published in July 2010. Also the status notes do not in all cases reflect the up to date situation. This is also being reviewed). The species listed here are those known to have bred in Arabia or are very likely to breed. The order follows the List of recent Holarctic bird species by K. H. Voous (published by the British Ornithologists Union, 1977) but certain changes have been made to bird names to accord with present usage. The reference number shown on the left is the number to be inserted in Column 2 of the ABBA Report sheet Form 3. The few breeding species not on the Voous list (these are mainly ferally breeding exotics) are given a separate sequence commencing with 2001. The Breeding Evidence Code (BEC) used by the ABBA project should be read in conjunction with this list. Details of species additional to the list, for which breeding is proven (BEC 10 or higher), will be published in future issues of Phoenix. Observers suspecting or proving breeding of species not shown on the list should make a full report. Highly sedentary species are identified with an asterisk (*). Because presence of these species, even outside the breeding season, is a good indication of local breeding. BEC "XX" may be used to report occurrence in suitable habitat at any time. -

CBD First National Report
First Saudi Arabian National Report on the Convention on Biological Diversity Chief Editor: Prof Dr Abdulaziz H. AbuZinada Editors: E.R. Robinson, Iyad A. Nader & Yousef I. Al Wetaid Publisher The National Commission for Wildlife Conservation and Development List of Contributors Chief Editor: Prof. Dr. Abdulaziz H. Abuzinada Editors: E.R. Robinson, Iyad H. Nader & Yousef I. Al Wetaid Organiser: Yousef I. Al Wetaid Contributors: Ahmad Al Mansi Eugene Joubert Faizi Shahul General Presidency of Meteorology and Environment Hashim Al Niaziy (Ministry of Economy and Planning) Hassan Mustafa King Abdulaziz City for Science and Technology Othman LLewellyn Ministry of Water and Electricity Ministry of Petroleum and Minerals Mohammed Al Toraif Mohammed O. Al Saghan (Ministry of Agriculture) Salih al Saghir Tariq Abbasi Wilhelm Buttiker Yousef Al Wetaid 2 Foreword His Royal Highness Prince Sultan bin Abdulaziz Second Deputy Premier, Minister of Defence and Inspector General Conservation of biological diversity and the sustainable use of the resources of the Earth are enshrined in Islamic law and principles. It is therefore fitting that in 2001 the Kingdom of Saudi Arabia became a signatory to the Convention on Biological Diversity that seeks to ensure the conservation of species and their habitats for all time. Biodiversity is itself a complex resource, but it differs from many other natural resources in that it is managed at local levels, but is subject to claims and influences that may operate on a global scale. Consequently, successful conservation of biodiversity requires involvement of all stakeholders, from the smallest local communities to the global community. It is this that makes the Convention on Biological Diversity so important. -
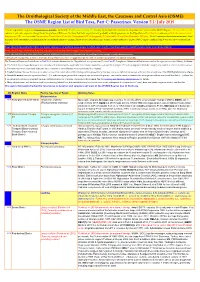
ORL 5.1 Passerines Final Draft01a.Xlsx
The Ornithological Society of the Middle East, the Caucasus and Central Asia (OSME) The OSME Region List of Bird Taxa, Part C: Passerines. Version 5.1: July 2019 A fuller explanation is given in Explanation of the ORL, but briefly, Bright green shading of a row (eg Syrian Ostrich) indicates former presence of a taxon in the OSME Region. Light gold shading in column A indicates sequence change from the previous ORL issue. For taxa that have unproven and probably unlikely presence, see the Hypothetical List. Red font indicates added information since the previous ORL version or the Conservation Threat Status (Critically Endangered = CE, Endangered = E, Vulnerable = V and Data Deficient = DD only). Not all synonyms have been examined. Serial numbers (SN) are merely an administrative convenience and may change. Please do not cite them in any formal correspondence or papers. NB: Compass cardinals (eg N = north, SE = southeast) are used. Rows shaded thus and with yellow text denote summaries of problem taxon groups in which some closely-related taxa may be of indeterminate status or are being studied. Rows shaded thus and with yellow text indicate recent or data-driven major conservation concerns. Rows shaded thus and with white text contain additional explanatory information on problem taxon groups as and when necessary. A broad dark orange line, as below, indicates the last taxon in a new or suggested species split, or where sspp are best considered separately. The Passerine Reference List follows as Part D, & includes References for Hypothetical non-passerines [List in Part E]. It explains Abbreviated References cited in the species accounts. -

International Council for Bird Preservation Biological Diversity
INTERNATIONAL COUNCIL FOR BIRD PRESERVATION BIOLOGICAL DIVERSITY ASSESSMENT OF THE REPUBLIC OF YEMEN by Daniel Martin Varisco, Team Leader James Perran Ross, Marine Biologist Anthony Milroy, Agricultural Engineer Editor Michael R. W. Rands, Programme Director, ICBP December 1990 ICBP 32 Cambridge Road Girton U.K.pCambridge CB3 OPJ DISCLAIMER This report wa researched and prepared before the reunification of the People's Democratic Republic of Yemen and the Yemen Arab Republic into the Republic of Yemen/' theretore only covers - 2 what was the Yemen Arab Republic (North Yemen), although much I 'i o of the information and some major recommendations may be equally appropriate to the whole country. CONTENTS EDITORIAL iv ACRONYMS USED IN THIS REPORT v EXECUTIVE SUMMARY vi 1. INTRODUCTION 1 1.1 Project rationale 1 1.2 Scope of work 1 1.3 Methodology 2 1.4 Status of available data 2 1.5 Background on Yemen's development context 3 2. BIOLOGICAL RESOURCES OF YEMEN 5 2.1 Ecosystem variety 5 2.2 Survey of flora 13 2.3 Survey of fauna 23 3. ENVIRON.AENTAL POLICY 32 3.1 Institutional responsibilities 32 3.2 Legislation regarding flora and forestry 33 3.3 Legislation regarding wildlife 34 3.4 Summary 34 4. ENVIRONMENTAL EDUCATION AND AWARENESS 35 4.1 Programs in government ministries 35 4.2 Media 36 4.3 NGO development 37 4.4 Summary 37 5. IMPACT OF DEVELOPMENT ON BIOLOGICAL RESOURCES 38 5.1 Overall development policy 38 5.2 Case study of Wadi Zabid 39 5.3 Integrated development policy and the envionrent 42 5.4 Coastal development 43 6.