The Association Between Hypocalcemia and Outcome in COVID-19 Patients: a Retrospective Study
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Clinical Physiology Aspects of Chloremia in Fluid Therapy: a Systematic Review David Astapenko1,2* , Pavel Navratil2,3, Jiri Pouska4,5 and Vladimir Cerny1,2,6,7,8,9
Astapenko et al. Perioperative Medicine (2020) 9:40 https://doi.org/10.1186/s13741-020-00171-3 REVIEW Open Access Clinical physiology aspects of chloremia in fluid therapy: a systematic review David Astapenko1,2* , Pavel Navratil2,3, Jiri Pouska4,5 and Vladimir Cerny1,2,6,7,8,9 Abstract Background: This systematic review discusses a clinical physiology aspect of chloride in fluid therapy. Crystalloid solutions are one of the most widely used remedies. While generally used in medicine for almost 190 years, studies focused largely on their safety have only been published since the new millennium. The most widely used solution, normal saline, is most often referred to in this context. Its excessive administration results in hyperchloremic metabolic acidosis with other consequences, including higher mortality rates. Methods: Original papers and review articles eligible for developing the present paper were identified by searching online in the electronic MEDLINE database. The keywords searched for included hyperchloremia, hypochloremia, and compound words containing the word “chloride,” infusion therapy, metabolic acidosis, renal failure, and review. Results: A total of 21,758 papers published before 31 May 2020 were identified; of this number, 630 duplicates were removed from the list. Upon excluding articles based on their title or abstract, 1850 papers were screened, of which 63 full-text articles were assessed. Conclusions: According to the latest medical concepts, dyschloremia (both hyperchloremia and hypochloremia) represents a factor indisputably having a negative effect on selected variables of clinical outcome. As infusion therapy can significantly impact chloride homeostasis of the body, the choice of infusion solutions should always take into account the potentially adverse impact of chloride content on chloremia and organ function. -

Hyponatremia in Hepatic Cirrhosis Following Paracentesis
HYPONATREMIA IN HEPATIC CIRRHOSIS FOLLOWING PARACENTESIS William P. Nelson III, … , Jack D. Rosenbaum, Maurice B. Strauss J Clin Invest. 1951;30(7):738-744. https://doi.org/10.1172/JCI102487. Research Article Find the latest version: https://jci.me/102487/pdf HYPONATREMIA IN HEPATIC CIRRHOSIS FOLLOWING PARACENTESIS 1 By WILLIAM P. NELSON, III, JACK D. ROSENBAUM, AND MAURICE B. STRAUSS (From the Medical Service, Cushing Veterans Administration Hospital, Framingham, Mass.) (Submitted for publication January 15, 1951; accepted April 23, 1951) The retention of water without a physiologically modification of the Folin procedure (14); non-protein equivalent amount of sodium following abdominal nitrogen by micro-Kjeldahl with Nesslerization (15); and sodium and potassium by means of the Barclay internal paracentesis has been studied in two patients with standard flame photometer. Except where otherwise advanced cirrhosis of the liver. In each there de- noted, urine was collected over 24 hour periods and was veloped manifestations considered characteristic analyzed for chloride by the Volhard-Harvey method of sodium deficit, although there was no change in (16), for total nitrogen by the micro-Kjeldahl procedure the total body sodium at the time these appeared. (11), and for creatinine, sodium, and potassium by the methods employed for serum. Change in total body water Such retention of water in excess of salt, regularly (liters) was taken to equal change in weight (kilograms). observed when large external losses of salt and water are replaced with water alone (1-3), has CASE REPORTS AND RESULTS been noted in certain cases of heart failure and chronic renal disease (4-6) as well as in decom- Case I. -

Hyperemesis Gravidarum with Paraparesis and Tetany
Open Access Case Report DOI: 10.7759/cureus.17014 Hyperemesis Gravidarum With Paraparesis and Tetany Jyotsnaa Muralitharan 1 , Vijayakumar Nagarajan 1 , Umarani Ravichandran 1 1. Internal Medicine, Rajah Muthiah Medical College & Hospital, Chidambaram, IND Corresponding author: Jyotsnaa Muralitharan, [email protected] Abstract Subacute-onset muscle weakness can result from channelopathies, inflammatory myopathies, thyroid dysfunction, hypoparathyroidism, vitamin D deficiency, and dyselectrolytemias like hypokalemia, hypocalcemia, and hypomagnesemia. We report a curious and extremely rare case of a 29-year-old woman with hyperemesis gravidarum presenting with disabling muscle weakness involving her lower limbs and trunk, and concurrent features of tetany. Following voluminous vomiting over the last two months, she presented with history of weakness of her lower limbs of 14 days duration, resulting in difficulty in her getting out of bed or walking unassisted. On examination, she was hypotensive (80/60 mmHg) and tachycardic (110 bpm), with flaccid weakness of her lower limbs (proximal weakness more than distal weakness - power of 1/5 at the hips bilaterally, and 3/5 at the knees and ankles bilaterally) and diminished deep tendon reflexes. She also had positive Trousseau’s sign and Chvostek’s sign. Interestingly, she also had thinned-out bluish sclerae, a high-arched palate, short stature, and bilateral conductive hearing loss. Laboratory evaluation revealed anemia, hyponatremia, hypokalemia, hypomagnesemia, hypochloremia, hypophosphatemia, and low vitamin D levels. Electrocardiogram showed prolonged QT interval. Her thyroid function test and parathyroid levels were normal. With parenteral replenishment of the electrolytes and vitamin D, her power improved and she was discharged on oral supplements. Thus, this case report demonstrates the importance of aggressive, early, and adequate management of hyperemesis gravidarum to prevent dyselectrolytemia-associated paraparesis. -

The Prognostic Effects of Hyponatremia and Hyperchloremia on Postoperative NSCLC Patients
Current Problems in Cancer 43 (2019) 402–410 Contents lists available at ScienceDirect Current Problems in Cancer journal homepage: www.elsevier.com/locate/cpcancer The prognostic effects of hyponatremia and hyperchloremia on postoperative NSCLC patients Wei Li a,b,1, Xiaowei Chen a,1, Liguang Wang a,c, Yu Wang a, ∗ Cuicui Huang a, Guanghui Wang a,d, Jiajun Du a,d, a Institute of Oncology, Shandong Provincial Hospital Affiliated to Shandong University, Jinan, PR China b Department of Thoracic Surgery, Shandong Juxian People’s Hospital, Rizhao, PR China c Department of Oncology, Shandong Provincial Hospital Affiliated to Shandong University, Jinan, PR China d Department of Thoracic Surgery, Shandong Provincial Hospital Affiliated to Shandong University, Jinan, PR China a b s t r a c t Electrolytic disorders are common in lung cancer patients. But the association between serum electrolytes levels and survival in patients undergoing lung cancer resections for non–small-cell lung cancer (NSCLC) has been poorly inves- tigated. A retrospective study was conducted on consecutive postoperative NSCLC patients. Pearson’s test was used to determine the association between serum sodium and chlorine levels and clinical characteristics, and cox regression and Kaplan-Meier model were applied to analyze risk factors on overall survival. We found that hyponatremia was an independent prognostic factor associated with poor prognosis in NSCLC patients undergoing complete resection (log-rank test, P = 0.004). In addition, we found that hyperchloremia predicted a poor clinical outcome in patients with non-anion-gap (log-rank test, P = 0.011), whereas it predicted a favorable clinical outcome in patients with high- anion-gap (log-rank test, P = 0.002). -

Calcinosis Cutis,Calcinosis Circumscripta,And “Mille Feuille
Calcinosis Cutis, Calcinosis Circumscripta, and “Mille Feuille” Lesions James Yi-Chien Lin, DVM MS; Han-Ju Tsai, DVM MS; Kau-Shen Hsu, DVM; Fun-In Wang, DVM PhD Skin and subcutaneous lesions of 2 cases with natural occurring Cushing’s disease and 1 case with calcinosis circumscripta were compared. Case 1 was typical of osteoma cutis, containing somewhat regularly arranged discrete ossification foci in the mid and deep dermis. Case 2 had layers of “mille feuille”, yellowish to white gritty chalky substances diffusely scattered in the sub- cutis, seen histologically as disseminatedly scattered light purple crystalloid and blue granular mineral salts. Lesions stained orange red with Alizarin Red S indicated the presence of calcium ions. Discrete ossification foci in the deep dermis and early multifocal collagenolysis with mine- ralization were also noted. Case 3 was typical of calcinosis circumscripta, seen grossly as yel- lowish chalky substance in both dermis and subcutis, and histologically as lakes of well- circumscribed light purple crystals and granular deep blue mineral salts. Case 2 had features of calcinosis cutis such as ossification foci and early multifocal collagenolysis. Case 2 also had “mille feuille” that was histologically similar to those mineral salts in case 3, but was not circum- scribed, and was not exactly calcinosis universalis. The component in case 1 was most likely hydroxyapatite Ca10(PO4)6(OH)2; the “mille feuille” of case 2 was most likely “calcium soap” after panniculitis and fat necrosis; and that in case 3 was most likely calcium phosphate CaPO4. Lo- cal factors, such as fluid exudation reflecting how well the inflammation was controlled clinically, may influence the wound healing, and thus the outcome of lesions. -

Sex Differences in Electrolyte Imbalances Caused by SARS-Cov-2
Sex differences in electrolyte imbalances caused by SARS-CoV-2: a case-control study Arianna Pani1, Elvira Inglese2, Massimo Puoti2, Valeria Cento1, Claudia Alteri1, Alessandra Romandini1, Federica Di Ruscio2, Michele Senatore1, Mauro Moreno2, Paolo Tarsia2, Fabrizio Colombo2, Oscar Epis2, Valentina Panetta3, Chiara Vismara2, Andrea Bellone2, and Francesco Scaglione1 1Universit`adegli Studi di Milano 2Niguarda Hospital 3L’altrastatistica srl - Consultancy & Training March 27, 2021 Abstract Background - Since SARS-CoV-2 spread, evidences regarding sex differences in progression and prognosis of COVID-19 have emerged. Besides this, studies on patients’ clinical characteristics have described electrolyte imbalances as one of the recurrent features of COVID-19. Methods - We performed a case-control study on all patients admitted to the emergency department (ED) from 1st March to 31st May 2020 who had undergone a blood gas analysis and a nasopharyngeal swab test for SARS- CoV-2 by rtPCR. We defined positive patients as cases and negatives as controls. The study was approved by the local ethics committee Area 3 Milan. Data were automatically extracted from the hospital laboratory SQL-based repository in anonymized form. We considered as outcomes potassium (K+), sodium (Na+), chlorine (Cl-) and calcium (Ca++) as continuous and as categorical variables, in their relation with age, sex and SARS-CoV-2 infection status. Results - We observed a higher prevalence of hypokalemia among patients positive for SARS-CoV-2 (13.7% vs 6% of negative subjects). Positive patients had a higher probability to be admitted to the ED with hypokalemia (OR 2.75, 95% CI 1.8-4.1 p<0.0001) and women were twice as likely to be affected than men (OR 2.43, 95% CI 1.67-3.54 p<0.001). -

NAME:ETINOSA-OGBAHON OSASENAGA PHARMACOLOGY 18/MHS07/019 BCH 204 Question 1
NAME:ETINOSA-OGBAHON OSASENAGA PHARMACOLOGY 18/MHS07/019 BCH 204 Question 1. Outline the toxicity values and deficiency manifestation of the following minerals: ● Potassium ● Calcium ● Magnesium ● Chloride ● Iron ◆POTASSIUM DEFICIENCY MANIFESTATION A. Hyperkalemia Hyperkalemia is a clinical condition associated with elevated plasma potassium above the normal range (3.5–5 mEq/L). Causes of hyperkalemia •Renal failure: The kidney may not be able to excrete a potassium load when GFR is very low. •Mineralocorticoid deficiency: For example, in Addison’s disease. •Cell damage: For example, in trauma and malignancy. Symptoms of hyperkalemia First manifestation is cardiac arrest, changes in electro- cardiogram, cardiac arrhythmia, muscle weakness which may be preceded by parasthesia (abnormal tingling sensation). Treatment for Hyperkalemia Treatment may include: •Going on a low-potassium diet •Stopping or changing meds that are contributing to the hyperkalemia •Taking medicine to lower the potassium in your body. Water pills (diuretics) remove potassium via the urinary tract. •Treating your kidney disease, which may include dialysis, which filters potassium from your blood B.Hypokalemia (low plasma concentration) In hypokalemia, the level of potassium in blood is too low. Causes of hypokalemia •Gastrointestinal losses: Potassium may be lost from the intestine due to vomiting, diarrhea. •Renal losses: Due to renal disease, administration of diuretics. •Drinking too much alcohol •Sweating a lot •Folic acid deficiency •Certain antibiotics •Diabetic ketoacidosis (high levels of acids called ketones in your blood) •Laxatives taken over a long period of time •Certain types of tobacco •Some asthma medications •Low magnesium Several syndromes can be associated with low potassium, such as: •Cushing's syndrome •Gitelman syndrome •Liddle syndrome •Bartter syndrome •Fanconi syndrome Symptoms of hypokalemia Muscular weakness, tachycardia, electrocardiographic (ECG) changes (flattering of ECG waves), lethargy, and confusion. -

Bartter Syndrome Precision Panel Overview Indications Clinical Utility
Bartter Syndrome Precision Panel Overview Bartter Syndrome is an autosomal recessive renal tubular disorder caused by a defective salt reabsorption in the thick ascending loop of Henle resulting in hypokalemia, hypochloremia, metabolic alkalosis, high renin and aldosterone with normal blood pressure. In neonatal cases, it can be suspected before birth and diagnosed soon after birth whereas in classic cases the presentation can begin at 2 years of age or younger. The genetic heterogeneity of this disease comes from genetic mutations in either the sodium chloride/potassium chloride cotransporter or the potassium channel transporter in the thick ascending loop of Henle. The Igenomix Bartter Syndrome Precision Panel can be used to make a directed and accurate diagnosis ultimately leading to a better management and prognosis of the disease. It provides a comprehensive analysis of the genes involved in this disease using next-generation sequencing (NGS) to fully understand the spectrum of relevant genes involved. Indications The Igenomix Bartter Syndrome Precision Panel is indicated for those patients with a clinical suspicion or diagnosis of Bartter Syndrome presenting with: - Family history of Bartter Syndrome - Maternal polyhydramnios - Fetal fluid loss and volume depletion - Failure to thrive - Polyuria - Polydipsia - Vomiting - Constipation - Salt craving Clinical Utility The clinical utility of this panel is: - The genetic and molecular confirmation for an accurate clinical diagnosis of a symptomatic patient. 1 - Early initiation of treatment with a multidisciplinary team in the form of medical care with potassium sparing diuretics, ion supplements and counselling regarding pregnancy- related considerations. - Risk assessment of asymptomatic family members according to the mode of inheritance. -

Issues in Endocrinology
VetEdPlus E-BOOK RESOURCES Issues in Endocrinology WHAT’S INSIDE The Diagnosis of Canine Hyperadrenocorticism Canine Hypothyroidism Feline Diabetes Mellitus Hypoadrenocorticism: Diagnosis and Treatment of Addison’s Disease Treatment of Pituitary-dependent A SUPPLEMENT TO Made possible by Hyperadrenocorticism an educational grant: Canine Diabetes Mellitus Chronic Pancreatitis in Felines E-BOOK PEER REVIEWED The Diagnosis of Canine Hyperadrenocorticism Audrey Cook, BVM&S, MSc VetEd, MRCVS, DACVIM-SAIM, DECVIM-CA, DABVP (Feline) Department of Small Animal Clinical Sciences, Texas A&M College of Veterinary Medicine and Biomedical Sciences College Station, Texas Hyperadrenocorticism (HAC or Cushing’s syndrome must have some (usually many) of the syndrome) describes the clinical manifestations classic signs (BOX 1). of chronic exposure to excessive glucocorticoids. Spontaneous HAC is often caused by More than 95% of dogs are polyuric/polydipsic; inappropriate secretion of adrenocorticotropic a normal water intake makes HAC less likely. hormone (ACTH) by a pituitary tumor (i.e., Additionally, most manifest dermatologic pituitary-dependent HAC [PDH]) or may reflect changes;2 in my experience, a good hair coat the autonomous production of cortisol by an adrenal tumor (AT).1 There are occasional reports of dogs with HAC BOX 1 Clinical Signs Commonly due to an aberrant response to a digestive hormone Associated With Canine HAC (i.e., food-dependent HAC) or from ectopic Polyuria and polydipsia ACTH secretion, but these are extremely rare. Polyphagia Panting CLINICAL PRESENTATION Abdominal distention Spontaneous HAC is usually diagnosed in older Hepatomegaly dogs, particularly Boston terriers, dachshunds, Muscle weakness 1 miniature poodles, and beagles. It is uncommon Dermatologic changes in dogs younger than 5 years of age. -

Genetics of Hereditary Disorders of Magnesium Homeostasis
Pediatr Nephrol (2004) 19:13–25 DOI 10.1007/s00467-003-1293-z REVIEW Karl P. Schlingmann · Martin Konrad · Hannsjrg W. Seyberth Genetics of hereditary disorders of magnesium homeostasis Received: 28 April 2003 / Revised: 25 July 2003 / Accepted: 28 July 2003 / Published online: 22 November 2003 IPNA 2003 Abstract Magnesium plays an essential role in many transporters and in signal transduction. Under physiolog- biochemical and physiological processes. Homeostasis of ical conditions, serum magnesium levels are maintained magnesium is tightly regulated and depends on the at almost constant values. Homeostasis depends on the balance between intestinal absorption and renal excretion. balance between intestinal absorption and renal excretion. During the last decades, various hereditary disorders of Magnesium deficiency can result from reduced dietary magnesium handling have been clinically characterized intake, intestinal malabsorption, or renal loss. The control and genetic studies in affected individuals have led to the of body magnesium homeostasis primarily resides in the identification of some molecular components of cellular kidney tubules. magnesium transport. In addition to these hereditary A number of acquired and hereditary disorders of forms of magnesium deficiency, recent studies have magnesium handling, most of them due to renal magne- revealed a high prevalence of latent hypomagnesemia in sium loss, have been described, all of them being the general population. This finding is of special interest relatively rare. The phenotypic characterization of clin- in view of the association between hypomagnesemia and ically affected individuals and experimental studies have common chronic diseases such as diabetes, coronary heart allowed the identification of the nephron segments disease, hypertension, and asthma. -

Electrolyte Abnormalities in Neonatesneonates
Electrolyte Abnormalities in NeonatesNeonates Jon Palmer, VMD, DACVIM Director of Neonatal/Perinatal Programs Graham French Neonatal Section, Connelly Intensive Care Unit New Bolton Center, University of Pennsylvania ElectrolyteElectrolyte abnormalities abnormalities CriticallyCritically ill ill neonates neonates • Frequently occur • Usually mild disturbances can be life-threatening • Epiphenomena Reflecting organ dysfunction • Gastrointestinal • Renal • Endocrine Reflecting global insult • Iatrogenic Fluid therapy errors Feeding mishaps • Fetal to neonatal physiology transition ElectrolyteElectrolyte Abnormalities Abnormalities • Sodium/Water Balance • Hyponatremia/Hypernatremia • Hypokalemia/Hyperkalemia • Hypocalcemia/Hypercalcemia • Hypomagnesemia/Hypermagnesemia Sodium/WaterSodium/Water Balance Balance • Transition from fetal physiology Late term fetus High FxNa Transition – to low FxNa • Most species during 1st day • Fetal foal - before birth • Sodium conserving mode Na requirement for growth • Bone growth • ↑body mass Increase in interstitial space Milk diet • Fresh milk is sodium poor 9-15 mEq/l Sodium/Water Balance SodiumSodium Conservation Conservation • Neonatal kidney less able to excrete Na load rapidly • ↓GFR • Glomerulotubular balance Absorption Na in proximal tubule balanced with snGFR Adult – distal tubule modulated based on Na balance Neonate – both proximal and distal tubules Distal important compensatory mechanism • Retention Na for growth • No autoregulation GFR at neonatal BP • Disruption Na reabsorption capacity -
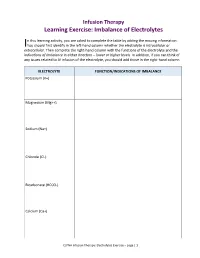
Learning Exercise: Imbalance of Electrolytes
Infusion Therapy Learning Exercise: Imbalance of Electrolytes n this learning activity, you are asked to complete the table by adding the missing information. I You should first identify in the left-hand column whether the electrolyte is intracellular or extracellular. Then complete the right-hand column with the functions of the electrolyte and the indications of imbalance in either direction – lower or higher levels. In addition, if you can think of any issues related to IV infusion of the electrolyte, you should add those in the right-hand column. ELECTROLYTE FUNCTION/INDICATIONS OF IMBALANCE Potassium (K+) Magnesium (Mg++) Sodium (Na+) Chloride (Cl-) Bicarbonate (HCO3-) Calcium (Ca+) CLPNA Infusion Therapy: Electrolytes Exercise – page | 1 Answers for Electrolyte Table Potassium (K+) (Intracellular) The distribution of potassium between the intracellular and extracellular compartments regulates electrical membrane potentials controlling the excitability of nerve and muscle cells as well as the contractility of skeletal, cardiac, and smooth muscle tissue. When levels of potassium are low (hypokalemia), signs and symptoms include dizziness, muscle weakness, leg cramps, cardiac arrhythmia, hypotension, thirst, nausea, anorexia, poorly concentrated urine, [and] polyuria. When levels of potassium are high (hyperkalemia), signs and symptoms include nausea and vomiting, intestinal cramps, diarrhea, paresthesias, weakness, dizziness, muscle cramps, changes in electrocardiogram, [and] risk of cardiac arrest with severe excess. Magnesium (Mg++) (Intracellular) Magnesium acts as a cofactor in many intracellular enzyme reactions…[and] is essential to all reactions that require ATP, for every step related to replication and transcription of DNA, and for the translation of messenger RNA…[and] is required for cellular energy metabolism.