Monitoring and Removal of Natural Organic Matter (NOM) from Toowoomba Water Sources
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Queensland Public Boat Ramps
Queensland public boat ramps Ramp Location Ramp Location Atherton shire Brisbane city (cont.) Tinaroo (Church Street) Tinaroo Falls Dam Shorncliffe (Jetty Street) Cabbage Tree Creek Boat Harbour—north bank Balonne shire Shorncliffe (Sinbad Street) Cabbage Tree Creek Boat Harbour—north bank St George (Bowen Street) Jack Taylor Weir Shorncliffe (Yundah Street) Cabbage Tree Creek Boat Harbour—north bank Banana shire Wynnum (Glenora Street) Wynnum Creek—north bank Baralaba Weir Dawson River Broadsound shire Callide Dam Biloela—Calvale Road (lower ramp) Carmilla Beach (Carmilla Creek Road) Carmilla Creek—south bank, mouth of creek Callide Dam Biloela—Calvale Road (upper ramp) Clairview Beach (Colonial Drive) Clairview Beach Moura Dawson River—8 km west of Moura St Lawrence (Howards Road– Waverley Creek) Bund Creek—north bank Lake Victoria Callide Creek Bundaberg city Theodore Dawson River Bundaberg (Kirby’s Wall) Burnett River—south bank (5 km east of Bundaberg) Beaudesert shire Bundaberg (Queen Street) Burnett River—north bank (downstream) Logan River (Henderson Street– Henderson Reserve) Logan Reserve Bundaberg (Queen Street) Burnett River—north bank (upstream) Biggenden shire Burdekin shire Paradise Dam–Main Dam 500 m upstream from visitors centre Barramundi Creek (Morris Creek Road) via Hodel Road Boonah shire Cromarty Creek (Boat Ramp Road) via Giru (off the Haughton River) Groper Creek settlement Maroon Dam HG Slatter Park (Hinkson Esplanade) downstream from jetty Moogerah Dam AG Muller Park Groper Creek settlement Bowen shire (Hinkson -

Hydrological Advice to Commission of Inquiry Regarding 2010/11 Queensland Floods
Hydrological Advice to Commission of Inquiry Regarding 2010/11 Queensland Floods TOOWOOMBA AND LOCKYER VALLEY FLASH FLOOD EVENTS OF 10 AND 11 JANUARY 2011 Report to Queensland Floods Commission of Inquiry Revision 1 12 April 2011 Hydrological Advice to Commission of Inquiry Regarding 2010/11 Queensland Floods TOOWOOMBA AND LOCKYER VALLEY FLASH FLOOD EVENTS OF 10 AND 11 JANUARY 2011 Revision 1 11 April 2011 Sinclair Knight Merz ABN 37 001 024 095 Cnr of Cordelia and Russell Street South Brisbane QLD 4101 Australia PO Box 3848 South Brisbane QLD 4101 Australia Tel: +61 7 3026 7100 Fax: +61 7 3026 7300 Web: www.skmconsulting.com COPYRIGHT: The concepts and information contained in this document are the property of Sinclair Knight Merz Pty Ltd. Use or copying of this document in whole or in part without the written permission of Sinclair Knight Merz constitutes an infringement of copyright. LIMITATION: This report has been prepared on behalf of and for the exclusive use of Sinclair Knight Merz Pty Ltd’s Client, and is subject to and issued in connection with the provisions of the agreement between Sinclair Knight Merz and its Client. Sinclair Knight Merz accepts no liability or responsibility whatsoever for or in respect of any use of or reliance upon this report by any third party. Toowoomba and the Lockyer Valley Flash Flood Events of 10 and 11 January 2011 Contents 1 Executive Summary 1 1.1 Description of Flash Flooding in Toowoomba and the Lockyer Valley1 1.2 Capacity of Existing Flood Warning Systems 2 1.3 Performance of Warnings -
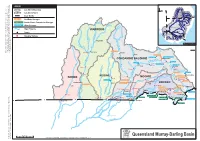
Queensland Murray-Darling Basin Catchments
LEGEND Catchment Boundary Charleville PAROO Catchment Name Roma Toowoomba St George State Border ondiwindi QUEENSLAND Go Leslie Dam SunWater Storages Brewarrina Glenlyon Dam Border Rivers Commission Storages Nygan Cooby Dam Other Storages Broken Hill Menindee NEW SOUTH WALES Major Streams SOUTH WARREGO AUSTRALIA Towns Canberra VICTORIA bury Gauging Stations Al ndigo Be Nive River Ward River Ward Augathella L Murray Darling Basin a Maranoa n g lo R Bungil Ck While every care is taken to ensure the accuracy of this product, Department Environment and Resource iv River er Neil Turner Weir Disclaimer: completeness or suitability for any particular reliability, Management makes no representations or warranties about its accuracy, purpose and disclaims all responsibility liability (including without limitation, in negligence) for expenses, losses, or incomplete in any way and for reason. damages (including indirect or consequential damage) and costs which you might incur as a result of the product being inaccurate Binowee Charleville Mitchell Roma Chinchilla Weir Creek Gilmore Weir Charleys Creek Weir Chinchilla CONDAMINE BALONNE k gw oo d C Warrego o D Warra Weir Surat Weir Brigalow Condamine Weir C River Creek o Loudon Weir reviR reviR Cotswold n Surat d Dalby a Wyandra Tara m r e i iv n Fairview Weribone e R Ck e Oak ey n Creek n o Cecil Weir Cooby Dam l a Wallen B Toowoomba Lemon Tree Weir NEBINE Cashmere River PAROO MOONIE Yarramalong Weir Cardiff R iv Tummaville Bollon Weir Beardmore Dam Moonie er Flinton River Talgai Weir Cunnamulla -

Final Report 1 July 2008 – 26 March 2009
Final Report 1 July 2008 – 26 March 2009 Department of Natural Resources and Water Published by: Department of Environment and Resource Management GPO Box 2454 Brisbane Qld 4001 © The State of Queensland (Department of Environment and Resource Management) 2009 The Department of Environment and Resource Management authorises the reproduction of textual material, whole or in part and in any form, provided appropriate acknowledgement is given. This report is available in alternative formats through Communication Services. Phone: +61 7 3227 8311 Email: [email protected]. If you need to access this report in a language other than English, please call the Translating and Interpreting Service (TIS National) on 131 450 and ask them to telephone Library Services on +61 7 3224 8412. This report may contain photos of Indigenous persons who are deceased. Sincere thanks to those who helped to prepare this report, including those who contributed information and images. Published August 2009 ISBN 931-1662183507 #28861 ii 28 August 2009 The Honourable Craig Wallace MP Minister for Natural Resources and Water Minister assisting the Premier in North Queensland Level 13, Mineral House 41 George Street Brisbane Qld 4000 Dear Minister I am pleased to present the Final Report 2008–09 for the Department of Natural Resources and Water. The functions of the former Department of Natural Resources and Water (NRW) were transferred to the new Department of Environment and Resource Management on 26 March 2009. This fi nal report outlines NRW’s activities and achievements up to the transfer date. I certify that this Final Report complies with: • the prescribed requirements of the Financial Accountability and Audit Act 1977 and the Financial Management Standard 1997, and • the detailed requirements set out in the Annual Reporting Guidelines for Queensland Government Agencies. -

DNRM Report Template Mixed with Numbers
Condamine–Balonne Water Resource Plan Provided for accreditation under Section 63 of the Water Act 2007 (Commonwealth) April 2019 This publication has been compiled by Water Policy South, Department of Natural Resources, Mines and Energy. © State of Queensland, 2019 The Queensland Government supports and encourages the dissemination and exchange of its information. The copyright in this publication is licensed under a Creative Commons Attribution 3.0 Australia (CC BY) licence. Under this licence you are free, without having to seek our permission, to use this publication in accordance with the licence terms. You must keep intact the copyright notice and attribute the State of Queensland as the source of the publication. Note: Some content in this publication may have different licence terms as indicated. For more information on this licence, visit http://creativecommons.org/licenses/by/3.0/au/deed.en The information contained herein is subject to change without notice. The Queensland Government shall not be liable for technical or other errors or omissions contained herein. The reader/user accepts all risks and responsibility for losses, damages, costs and other consequences resulting directly or indirectly from using this information. Table of contents Introduction ......................................................................................................................... 6 The Condamine–Balonne Water Resource Plan area ..................................................... 7 2.1 WRP area ............................................................................................................................ -

An Economic Assessment of the Value of Recreational Angling at Queensland Dams Involved in the Stocked Impoundment Permit Scheme
An economic assessment of the value of recreational angling at Queensland dams involved in the Stocked Impoundment Permit Scheme Daniel Gregg and John Rolfe Value of recreational angling in the Queensland SIP scheme Publication Date: 2013 Produced by: Environmental Economics Programme Centre for Environmental Management Location: CQUniversity Australia Bruce Highway North Rockhampton 4702 Contact Details: Professor John Rolfe +61 7 49232 2132 [email protected] www.cem.cqu.edu.au 1 Value of recreational angling in the Queensland SIP scheme Executive Summary Recreational fishing at Stocked Impoundment Permit (SIP) dams in Queensland generates economic impacts on regional economies and provides direct recreation benefits to users. As these benefits are not directly traded in markets, specialist non-market valuation techniques such as the Travel Cost Method are required to estimate values. Data for this study has been collected in two ways in 2012 and early 2013. First, an onsite survey has been conducted at six dams in Queensland, with 804 anglers interviewed in total on their trip and fishing experiences. Second, an online survey has been offered to all anglers purchasing a SIP licence, with 219 responses being collected. The data identifies that there are substantial visit rates across a number of dams in Queensland. For the 31 dams where data was available for this study, recreational anglers purchasing SIP licences have spent an estimated 272,305 days fishing at the dams, spending an average 2.43 days per trip on 2.15 trips per year to spend 4.36 days fishing per angler group. Within those dams there is substantial variation in total fishing effort, with Somerset, Tinaroo, Wivenhoe and North Pine Dam generating more than 20,000 visits per annum. -

Fisheries (Freshwater) Management Plan 1999
Queensland Fisheries Act 1994 Fisheries (Freshwater) Management Plan 1999 Reprinted as in force on 13 June 2008 Reprint No. 3B This reprint is prepared by the Office of the Queensland Parliamentary Counsel Warning—This reprint is not an authorised copy Information about this reprint This plan is reprinted as at 13 June 2008. The reprint shows the law as amended by all amendments that commenced on or before that day (Reprints Act 1992 s 5(c)). The reprint includes a reference to the law by which each amendment was made—see list of legislation and list of annotations in endnotes. Also see list of legislation for any uncommenced amendments. This page is specific to this reprint. See previous reprints for information about earlier changes made under the Reprints Act 1992. A table of reprints is included in the endnotes. Also see endnotes for information about— • when provisions commenced • editorial changes made in earlier reprints. Spelling The spelling of certain words or phrases may be inconsistent in this reprint due to changes made in various editions of the Macquarie Dictionary. Variations of spelling will be updated in the next authorised reprint. Dates shown on reprints Reprints dated at last amendment All reprints produced on or after 1 July 2002, authorised (that is, hard copy) and unauthorised (that is, electronic), are dated as at the last date of amendment. Previously reprints were dated as at the date of publication. If an authorised reprint is dated earlier than an unauthorised version published before 1 July 2002, it means the legislation was not further amended and the reprint date is the commencement of the last amendment. -

Tillegra Dam Effects on Dungog Council Road Network Infrastructure
Tillegra Dam Effects on Dungog Council Road network infrastructure Greg McDonald September 2007 Amended July 2008 1 Pavement Design Basics Road pavements are designed for a finite life. When the designer needs to determine a design life for the road they typically choose a period of approximately 20 to 30 years. Having a design life, the designer then needs to determine the number of Equivalent Standard Axles (ESAs) that the road is anticipated to carry in that design period, and this figure can then be used to determine a pavement thickness. A ‘Standard Axle’ is a design equivalent to enable various differently loaded axles to be factored into the pavement design. It takes into effect the load and axle configuration of heavy vehicles. The standard axle in which all others are related is a single axle load of 8.2 tonnes (80 kN) on dual tyres. For vehicles that have different axle loadings, the equivalent loading can be calculated from the following formula: 4 NESA = [Pc / PESA) Where: Pc load on axle group PESA load on standard axle group For a motor car of approximately 1.6 tonne (standard Falcon or Commodore) the load on each axle group is approximately 800 kg. 4 NESA = (0.9/8.2) = 0.14 = 0.0001 Tillegra Dam – Road Improvements 2 (or 1 10,000th of an equivalent standard axle) So 1 car is equivalent to about 10,000 trucks! Because cars are so insignificant when compared to heavy vehicles, the design method for calculating total ESAs ignores normal traffic and only utilizes the heavy vehicle traffic. -

View Final Report 4.30 Mb
Environment and Natural Resources Committee Inquiry into Melbourne’s Future Water Supply June 2009 Inquiry into Melbourne’s Future Water Supply Report of the Environment and Natural Resources Committee on the Inquiry into Melbourne’s Future Water Supply ORDERED TO BE PRINTED Victorian Government Printer 2009 Parliamentary Paper No. 174 Session 2006-2009 i Parliament of Victoria Environment and Natural Resources Committee Inquiry into Melbourne’s Future Water Supply ISBN - 978-0-9757811-4-2 The Committee would like to thank Melbourne Water for allowing its images to be used on the front cover This report is printed on 100% recycled paper. Table of contents Committee Members ........................................................................................... xi The Environment and Natural Resources Committee .................................... xiii Terms of reference ............................................................................................. xv Chair’s foreword ............................................................................................... xvii Executive summary........................................................................................... xix Framework for managing Melbourne’s water supply ..................................... xix Water use efficiency and conservation ............................................................ xx Stormwater and rainwater – harvesting, storage and use ............................ xxii The reuse of treated wastewater .................................................................... -

Table of Contents FFSAQ’S News Items 2019
Table of Contents FFSAQ’S News Items 2019 FFSAQ News ..click on month January 2019 ..................................................................................... 2 March 2019 ...................................................................................... 11 April 2019 ......................................................................................... 16 May 2019 ......................................................................................... 21 July 2019 .......................................................................................... 26 August 2019 ..................................................................................... 31 June 2019 ......................................................................................... 36 September 2019 .............................................................................. 41 October 2019 ................................................................................... 45 November 2019 ............................................................................... 50 Press Home Key or Cntrl Home To return to this index FFSAQ News January 2019 Media Officer - Lloyd Willmann [email protected] Ph. 0429 614892 From the Media Officer's Desk :- Freshwater Workshop: DAF are still waiting for some delegates to complete the survey on the Warwick workshop. Your response will allow DAF and FFSAQ to tailor future workshops to your needs. Stocking Groups ... could you please ask your delegate/s to complete the survey (if they haven't done so already) -

Water Reuse and Recycling in Australia- History, Current Situation and Future Perspectives
Water Cycle 1 (2020) 19–40 Contents lists available at ScienceDirect Water Cycle journal homepage: www.keaipublishing.com/en/journals/water-cycle/ Water reuse and recycling in Australia- history, current situation and future perspectives John C. Radcliffe *, Declan Page CSIRO, Locked Bag 2, Glen Osmond, South Australia, 5064, Australia ARTICLE INFO ABSTRACT Keywords: Most of Australia has low rainfall. The population is small (25 M) but growing at 1.5%/yr. Water limitations are Desalination being exacerbated by climate change. By 1990, restraints placed on wastewater treatment plant (WWTP) dis- Drought charges to receiving waters became an incentive for water recycling. The millennium drought (2000–2009) was a Managed aquifer recharge further driver for water recycling and desalination. Water reform policies, led to guidelines for recycled water, Stormwater including stormwater and augmentation of drinking water. Advanced purified recycled water plants for indirect Water recycling potable reuse were built in Brisbane. Dual pipes for drinking and recycled water were installed in new suburbs in Sydney, Melbourne and Adelaide. Agricultural, industrial and amenity recycled water use was expanded. Seawater desalination plants were installed in Gold Coast, Sydney, Melbourne, Adelaide and Perth. After the drought, economics further influenced the future use of recycled water. Since catchment water was cheaper than recycled or desalinated water, desalination plants were mothballed or maintained at low processing rates. and Brisbane’s advanced water treatment plants were shut. Water policy complacency followed. However, Western Australia, which had declining rainfall, demonstrated to an accepting community that recycled water could be used for groundwater replenishment in Perth’s water supply. -

Final Sitting Week of the Year
PROOF ISSN 1322-0330 RECORD OF PROCEEDINGS Hansard Home Page: http://www.parliament.qld.gov.au/hansard/ E-mail: [email protected] Phone: (07) 3406 7314 Fax: (07) 3210 0182 Subject FIRST SESSION OF THE FIFTY-THIRD PARLIAMENT Page Thursday, 12 November 2009 SPEAKER’S STATEMENT ............................................................................................................................................................ 3337 Parliament House Events ................................................................................................................................................... 3337 PETITIONS ..................................................................................................................................................................................... 3337 TABLED PAPERS .......................................................................................................................................................................... 3337 MINISTERIAL PAPERS ................................................................................................................................................................. 3337 MINISTERIAL STATEMENTS ........................................................................................................................................................ 3338 Water Supply .....................................................................................................................................................................