Dynamic Covalent Polymers for Biomedical Applications Cite This: Mater
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Aalseth Aaron Aarup Aasen Aasheim Abair Abanatha Abandschon Abarca Abarr Abate Abba Abbas Abbate Abbe Abbett Abbey Abbott Abbs
BUSCAPRONTA www.buscapronta.com ARQUIVO 35 DE PESQUISAS GENEALÓGICAS 306 PÁGINAS – MÉDIA DE 98.500 SOBRENOMES/OCORRÊNCIA Para pesquisar, utilize a ferramenta EDITAR/LOCALIZAR do WORD. A cada vez que você clicar ENTER e aparecer o sobrenome pesquisado GRIFADO (FUNDO PRETO) corresponderá um endereço Internet correspondente que foi pesquisado por nossa equipe. Ao solicitar seus endereços de acesso Internet, informe o SOBRENOME PESQUISADO, o número do ARQUIVO BUSCAPRONTA DIV ou BUSCAPRONTA GEN correspondente e o número de vezes em que encontrou o SOBRENOME PESQUISADO. Número eventualmente existente à direita do sobrenome (e na mesma linha) indica número de pessoas com aquele sobrenome cujas informações genealógicas são apresentadas. O valor de cada endereço Internet solicitado está em nosso site www.buscapronta.com . Para dados especificamente de registros gerais pesquise nos arquivos BUSCAPRONTA DIV. ATENÇÃO: Quando pesquisar em nossos arquivos, ao digitar o sobrenome procurado, faça- o, sempre que julgar necessário, COM E SEM os acentos agudo, grave, circunflexo, crase, til e trema. Sobrenomes com (ç) cedilha, digite também somente com (c) ou com dois esses (ss). Sobrenomes com dois esses (ss), digite com somente um esse (s) e com (ç). (ZZ) digite, também (Z) e vice-versa. (LL) digite, também (L) e vice-versa. Van Wolfgang – pesquise Wolfgang (faça o mesmo com outros complementos: Van der, De la etc) Sobrenomes compostos ( Mendes Caldeira) pesquise separadamente: MENDES e depois CALDEIRA. Tendo dificuldade com caracter Ø HAMMERSHØY – pesquise HAMMERSH HØJBJERG – pesquise JBJERG BUSCAPRONTA não reproduz dados genealógicos das pessoas, sendo necessário acessar os documentos Internet correspondentes para obter tais dados e informações. DESEJAMOS PLENO SUCESSO EM SUA PESQUISA. -

Industry at Eurocmr 2019
EuroCMR2019 EXTENDING THE CLINICAL VALUE OF CMR THROUGH QUALITY AND EVIDENCE 2-4 May Venice ITALY FINAL PROGRAMME 17th Annual Meeting on Cardiovascular Magnetic Resonance (CMR) of the European Association of Cardiovascular Imaging (EACVI) www.escardio.org/EACVI #EuroCMR Programme as of 5 April 2019 Building overview EACVI MEMBERS’ LOUNGE COURSE ROOM MEETING ROOMS POSTERS Level 1 SPEAKER SERVICE CENTRE SALA DARSENA ENTRANCE SALA DARSENA SALA GRANDE Level 0 EXHIBITION REGISTRATION MAIN ENTRANCE Level -1 RAPID FIRE ROOM SALA ZORZI Welcome Letter Dear Friends & Colleagues, Welcome to EuroCMR 2019 Congress in Venice, Italy! We are delighted to welcome you to EuroCMR 2019, the annual meeting on CMR of the European Association of Cardiovascular Imaging (EACVI), which has developed over the last few years into the largest European CMR conference with over 1,000 participants in Prague in 2017. This year’s congress theme is “Extending the clinical value of CMR through quality and evidence”. Throughout the programme, we will focus on the existing and emerging evidence behind the established and potential clinical indications of CMR and will highlight how high-quality research and clinical service provision can make a difference to patients and populations. In times of very limited resources available to finance health care, we will also discuss health economics relevant to cardiac imaging in a multi-modality context. We want this to be your conference. We invited all members of the EACVI to actively contribute to EuroCMR 2019 by responding to our call for session proposals and we look forward to providing an international platform for you to present your research abstracts. -

Author Index
ABSTRACT AUTHOR INDEX The number following the name refers to the abstract number, not the page number. A number in bold indicates the author is the abstract presenter. Aaboe, Kasper 1915-P Adams, Rachel 2308-PUB, 2315-PUB Akhmetova, Saule B. 644-P, 649-P Aadahl, Mette 1997-P Adams, Sara R. 837-P, 1214-P Akhrass, Firas 1250-P Aamodt, Kristie I. 2056-P Adamson, Karen 1036-P, 1123-P, 1164-P, 1170-P Aki, Nanako 2412-PUB Abate, Nicola 33-OR Adeli, Khosrow 103-OR, 106-OR Akiyama, Noriko 1102-P Abbas, Malak 494-P, 2084-P Adhami, Anwar 539-P Akiyama, Yoshitaka 976-P Abbasi, Fahim 2140-PUB Adhikari, Pramisha 1726-P Akkari, Patrick Anthony 1613-P Abbass, Ibrahim 844-P, 2159-PUB Adhikary, Till 1850-P Akkireddy, Padmaja 2236-PUB Abbott, Akshar B. 608-P Adi, Saleh 873-P Akolkar, Beena 819-P Abbott, Alice M. 222-OR Adili, Fatemeh 2194-PUB Akoumianakis, Ioannis 215-OR Abbott, Scott 993-P, 1000-P, 1001-P, 1007-P Adkins, Laura 417-P Akturk, Halis K. 1586-P Abburi, Nandini 6-OR Advani, Andrew 485-P, 501-P, 552-P, 670-P Akueson, Cecelia E. 1347-P Abcouwer, Steven F. 601-P Aeby, Sébastien 766-P Al Jobori, Hussein 1097-P, 1875-P ABSTRACT AUTHOR INDEX Abdallah Hasan, Ahmed 505-P Aerni, Hans R. 1754-P Al Khaldi, Rasha 413-P, 2165-PUB Abdella, Nabilla 413-P, 2165-PUB Affi nati, Alison 2457-PUB Ala-Korpela, Mika 198-OR Abdesselam, Ines 2018-P Afrin, M.S.T. Rejina 2295-PUB Alarcón, Javier 1672-P Abdul-Ghani, Muhammad 188-OR, 1097-P, 1961-P Agarwal, Rishi 943-P Alarcon, Maria Lis 2338-PUB, 2362-PUB Abdulina, Galiya A. -
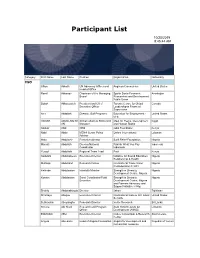
Participant List
Participant List 10/20/2019 8:45:44 AM Category First Name Last Name Position Organization Nationality CSO Jillian Abballe UN Advocacy Officer and Anglican Communion United States Head of Office Ramil Abbasov Chariman of the Managing Spektr Socio-Economic Azerbaijan Board Researches and Development Public Union Babak Abbaszadeh President and Chief Toronto Centre for Global Canada Executive Officer Leadership in Financial Supervision Amr Abdallah Director, Gulf Programs Educaiton for Employment - United States EFE HAGAR ABDELRAHM African affairs & SDGs Unit Maat for Peace, Development Egypt AN Manager and Human Rights Abukar Abdi CEO Juba Foundation Kenya Nabil Abdo MENA Senior Policy Oxfam International Lebanon Advisor Mala Abdulaziz Executive director Swift Relief Foundation Nigeria Maryati Abdullah Director/National Publish What You Pay Indonesia Coordinator Indonesia Yussuf Abdullahi Regional Team Lead Pact Kenya Abdulahi Abdulraheem Executive Director Initiative for Sound Education Nigeria Relationship & Health Muttaqa Abdulra'uf Research Fellow International Trade Union Nigeria Confederation (ITUC) Kehinde Abdulsalam Interfaith Minister Strength in Diversity Nigeria Development Centre, Nigeria Kassim Abdulsalam Zonal Coordinator/Field Strength in Diversity Nigeria Executive Development Centre, Nigeria and Farmers Advocacy and Support Initiative in Nig Shahlo Abdunabizoda Director Jahon Tajikistan Shontaye Abegaz Executive Director International Insitute for Human United States Security Subhashini Abeysinghe Research Director Verite -
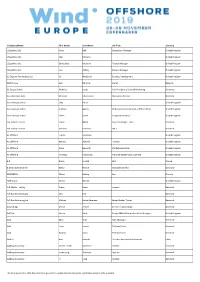
Company Name First Name Last Name Job Title Country
Company Name First Name Last Name Job Title Country 1StopWind Ltd Arran Bell Operations Manager United Kingdom 1StopWind Ltd. Alan Mckerns United Kingdom 1StopWind Ltd. Bernadette McAulay Finance Manager United Kingdom 1StopWind Ltd. Joel Telling General Manager United Kingdom 23 Degrees Renewables Ltd Ed Woodrow Business Development United Kingdom 24SEA bvba Gert De Sitter Owner Belgium 3S Europe GmbH Matthias Lamp Vice President of Sales & Marketing Germany 3sun Denmark ApS Christian Christensen Operations Director Denmark 3sun Group Limited Jody Potter United Kingdom 3sun Group Limited Graham Hacon VP Business Development, Offshore Wind United Kingdom 3sun Group Limited Sherri Smith Company Secretary United Kingdom 3W Industri Service Simon Øland Project manager - sales Denmark 3W Industri Service Kenneth Pedersen IWI-S Denmark 4C Offshore Lauren Anderson United Kingdom 4C Offshore Richard Aukland Director United Kingdom 4C Offshore Rosie Haworth Market Researcher United Kingdom 4C Offshore Vincenzo Poidomani Principal Geotechnical Engineer United Kingdom 8.2 Bruno ALLAIN CEO France 8.2 Monitoring GmbH Bernd Höring Managing director Germany 920338402 Ellinor Meling Ceo Norway A&P Group Emma Harrick United Kingdom A.P. Møller Holding Simon Ibsen Investor Denmark A/S Dan-Bunkering Ltd. Jens Kirk Denmark A/S Dan-Bunkering Ltd. Michael Brunø-Sørensen Senior Bunker Trader Denmark A1wind Aps Martin Jensen Director / A1wind Aps Denmark AAF Ltd Steven Brett Europe MFAS Aftermarket Sales Manager United Kingdom AAG Allan Tarp Sales Manager Denmark -

Müller-Weiss Disease: a Topical Review
FAIXXX10.1177/1071100719877000Foot & Ankle InternationalAhmed et al 877000research-article2019 Topical Review Foot & Ankle International® 2019, Vol. 40(12) 1447 –1457 Müller-Weiss Disease: A Topical Review © The Author(s) 2019 Article reuse guidelines: sagepub.com/journals-permissions DOI:https://doi.org/10.1177/1071100719877000 10.1177/1071100719877000 journals.sagepub.com/home/fai Abdel-Salam Abdel-Aleem Ahmed, MD1 , Mahmoud Ibrahim Kandil, MD1 , Eslam Abdelshafi Tabl, MD1 , and Amr S. Elgazzar, MD1 Level of Evidence: Level V, expert opinion. Keywords: Müller-Weiss disease, Brailsford disease, foot arthrodesis, calcaneal osteotomy Müller-Weiss disease (MWD) is a painful foot condition articulates proximally with the talar head, distally with the characterized by deformity, sclerosis, and fragmentation of cuneiforms, and inferolaterally with the cuboid. Proximally, the navicular. The diseased navicular is characteristically the talocalcaneonavicular articulation is also known as the comma shaped with varying degrees of arthritis present at acetabulum pedis for morphologic similarity to the hip the talonavicular and naviculocuneiform joints.17,19,27 Other joint. It is a ball-socket synovial joint allowing for rotatory names for MWD include Brailsford disease,37 adult tarsal and gliding movements. The talocalcaneonavicular and the scaphoiditis,26 spontaneous adult navicular osteonecrosis,34 naviculocuneiform joints sustain the greatest load transmis- and listhesis navicularis.23 There is substantial controversy sion in comparison to other foot joints.11 regarding the etiology, pathophysiology, and natural history The navicular is the latest tarsal bone to ossify. of MWD, as well as optimal methods of treatment. This Ossification occurs during the period of increased mobility article reviews the historical and current literature regarding and activity in children (at the end of the second year in girls MWD, including the latest surgical interventions that have and at the beginning of the fourth year in boys). -

ICCA Congress & Exhibition 2007
ICCA Congress & Exhibition 2007 PARTICIPANTS BY SURNAME 2007 Name Company Country , Suharyanto Jakarta Convention & Exhibition Bureau INDONESIA , Farizaludin Jakarta Convention & Exhibition Bureau INDONESIA ABDUL HALIM, Kamilia Malaysia Tourism Promotion Board MALAYSIA Hani ABDUL RAHMAN, Nina Kuala Lumpur Convention Centre MALAYSIA Kartini Bte ABDUL RAHMAN, Malaysia Tourism Promotion Board MALAYSIA Norshamshida ABDUL WAHID, Malaysia Tourism Promotion Board MALAYSIA Norhaida ABU MONASSAR, Anwar Net Conference & Conventions UNITED ARAB EMIRATES ADAMJI, Tasneem African Quest Safaris Ltd. KENYA ADAMS, Barbara Melbourne Exhibition and Convention Centre AUSTRALIA AKKANEEVANICH, Queen Sirikit National Convention Center THAILAND Sasivee ALAGIROVA, Tamara Bedouk Meetings & Events Media FRANCE AL FAHIM, Khalid Dubai International Convention & Exhibition Centre UNITED ARAB EMIRATES AL FALASI, Sultan Dubai Convention Bureau UNITED ARAB EMIRATES AL NUAIMI, Mubarak Abu Dhabi Tourism Authority UNITED ARAB EMIRATES ALONSO, Francisco ExpoChihuahua MEXICO ALTAMURA, Carrie Darwin Convention Centre AUSTRALIA ALTER, Alex ICC Jerusalem International Convention Center ISRAEL ALVEZ SARAVIA, CMP, ICCA Latin America Regional Office URUGUAY Maria José AMARAL, Jose Rui International Conference Center Joaquim Chissano MOZAMBIQUE AMEY, Monique Nice Convention & Visitors Bureau FRANCE AMORNVIVAT, Natwut Thailand Convention & Exhibition Bureau THAILAND AMSEL, Clare Megaron Athens International Conference Centre GREECE ANDERSEN, Peder DIS Congress Service Copenhagen A/S -
Toledo Airbrush Artist Brings Unique Style to the Diamond
$1 Mid-Week Edition Thursday, March 31, 2016 Serving our communities since 1889 — www.chronline.com Prep Baseball Roundup / Sports Brunswig’s Business Legislature Adjourns Family Shoe Store Has More Than a Centralia Lawmaker Lauds a Bipartisan Century of History in Chehalis / Main 3 Compromise on State Budget / Main 7 Woman Pleads Guilty to Burglary at Prosecutor’s House JAILED: Sentencing of burglarizing county Prosecu- Janet Lynn Gleason, 43, of to one count of possession of Meyer said he would rath- tor Jonathan Meyer’s home last Centralia, was charged on Oct. methamphetamine with intent er people come to him when Scheduled for May 11; year pleaded guilty this week in 29 in Lewis County Superior to deliver, from an unrelated ar- they’re angry than target his Burglar Faces 7 Years Lewis County Superior Court. Court with residential burglary, rest in September. family. She could be sentenced to as first-degree trafficking in sto- Meyer said Wednesday that “I’m glad it’s finally over,” By Natalie Johnson much as 84 months, or 7 years, in len property and second-degree he was happy someone would be Jonathan Meyer’s Wife, Mi- [email protected] prison at her sentencing hearing malicious mischief. held accountable in the case. chelle Meyer, said. on May 11, said Special Prosecutor On Wednesday, she plead- “It’s a little surreal being in A Centralia woman accused Mark McClain, of Pacific County. ed guilty to those charges and this position,” he said. please see BURGLARY, page Main 14 Lewis County Toledo Airbrush Artist Brings Superior Court Judge Unique Style to the Diamond Richard Brosey Announces Retirement By The Chronicle Lewis County Superior Court Judge Richard Brosey announced Wednesday that he plans to retire at the end of his current term, which ends in Jan- uary 2017. -

Characteristics, Prevention, and Management of Cardiovascular Disease in People Living with HIV: a Scientific Statement From
Circulation AHA SCIENTIFIC STATEMENT Characteristics, Prevention, and Management of Cardiovascular Disease in People Living With HIV A Scientific Statement From the American Heart Association ABSTRACT: As early and effective antiretroviral therapy has become more Matthew J. Feinstein, widespread, HIV has transitioned from a progressive, fatal disease to a MD, MSc, FAHA, Chair chronic, manageable disease marked by elevated risk of chronic comorbid Priscilla Y. Hsue, MD, Vice diseases, including cardiovascular diseases (CVDs). Rates of myocardial Chair infarction, heart failure, stroke, and other CVD manifestations, including Laura A. Benjamin, PhD pulmonary hypertension and sudden cardiac death, are significantly Gerald S. Bloomfield, MD, higher for people living with HIV than for uninfected control subjects, MPH, FAHA even in the setting of HIV viral suppression with effective antiretroviral Judith S. Currier, MD therapy. These elevated risks generally persist after demographic and Matthew S. Freiberg, MD, MSc clinical risk factors are accounted for and may be partly attributed to Steven K. Grinspoon, MD chronic inflammation and immune dysregulation. Data on long-term Jules Levin, MS Downloaded from http://ahajournals.org by [email protected] on June 3, 2019 CVD outcomes in HIV are limited by the relatively recent epidemiological Chris T. Longenecker, MD, transition of HIV to a chronic disease. Therefore, our understanding FAHA of CVD pathogenesis, prevention, and treatment in HIV relies on large Wendy S. Post, MD, MS observational studies, -
Louis Riel, Justice, and Mtis Self-Identification: Literary Politics
Robin White Goldsmiths College, University of London Thesis for the Degree of Ph.D., 2014 Louis Riel, Justice, and Métis Self-Identification: Literary Politics for Survival in the Evolution of Canadian Nationhood 1 I, Robin White, declare this thesis to be entirely my own original work. 2 Abstract of Thesis This thesis explores Métis identity in the context of the ambiguous nature of the Canadian national identity, and in particular, how the Louis Riel ‘Rebellions’ of 1869- 70 and 1885, continue to resonate in contemporary Métis literature. While it is the case that the Métis have self-identified within their own communities since the seventeenth century, it is also the case that the legal definition and recognition of the Métis were not realized until 1982. Indeed, the fine points of law in the Canadian Constitution are still in dispute, not least because the Métis were not included in the initial signing of treaties with the Dominion of Canada. For the purposes of this thesis, it is first necessary to engage in a thorough examination of historical accounts of the resistance of the Métis against expansionist encroachment of the Dominion of Canada in the Northwest in order to trace the source and evolution of contemporary Métis literature. I consider the ways in which the literary texts of Métis authors confront racial and cultural prejudice that are the direct result of Constitutional law, charters of rights, legislation in the judicial system, and the eleven Confederation numbered treaties, drafted before, during and after the Riel Resistances. The introductory and first chapters include contemporary Métis historical and legal academic analyses of the ‘Rebellions’ and an exploration of how the Government of Canada’s response in 1885 to the Métis uprising continues to inform its legal positions concerning Métis land rights and self-identification, while striving to maintain an international reputation as politically, and ethically, just. -
Download MSSP ACO Provider List (PDF)
MSSP ACO 2021 Particpating Providers As of June 1, 2021 ACO ACO Participating Provider Participating Provider Practice Location Practice Location Last Name First Name Street Address Line1 Street Address Line2 City State Zip Code Aaronson Emily 55 Fruit St. WHT 1 Boston MA 02114-2696 Abadir Matthew 55 Fruit St. Boston MA 02114 Abate Christina 10 Centennial Dr. Peabody MA 01960 Abbasi Bardia 243 Charles St. Boston MA 02114-3002 Abbate Lauren 294 Washington St. DTN 210 Boston MA 02108 Abbondanza Lisa 1280 West Central St. Ste. 201 Franklin MA 02038 Abbott Brian 32 Fruit St. YAW-03-3072 Boston MA 02114 Abbott Cara 65 Calef Highway Ste. 200 Lee NH 03861 Abbott Gerald 55 Fruit St. FND 202 Boston MA 02114-2696 Abbott Timothy 30 Locust St. Northampton MA 01060 Abbott Todd 2014 Washington St. Newton MA 02462 Abdar Esfahani Shadi 55 Fruit St. WHT 270 Boston MA 02114-2696 Abdi Reza 45 Francis St. Boston MA 02115 Abdul-Aziz Dunia 243 Charles St. Boston MA 02114 Abdulnour Raja-Elie 75 Francis St. PBB Clinics 3 Boston MA 02115 Abel Lea 75 Francis St. Boston MA 02115 Abel Scott One Parkway Haverhill MA 01830 Abela Andrew 100 Highland Ave. Salem MA 01970 Abele Michael One Parkway Haverhill MA 01830 Abelowitz Nina 133 ORNAC Ste. 200 Concord MA 01742 Abensohn Mark 266 Main St. Ste. 4 Medfield MA 02052 Aboobakar Inas 243 Charles St. Boston MA 02114 Abookire Susan 75 Francis St. Suite G. Boston MA 02115-6110 Abraczinskas Diane 165 Cambridge St. 9th Fl. Boston MA 02114-2783 Abraham Jonathan 75 Francis St. -
Exercise Training, Bone and Aging. Evidence from Different Impact Loading Exercise Interventions on Age-Related Bone Loss
Exercise training, Bone and Aging. Evidence from different impact loading exercise interventions on age-related bone loss. Elisa Amélia Alves Fernandes Marques The present dissertation was written in Dissertação apresentada com vista à order to achieve the PhD degree included in obtenção do grau de Doutor no âmbito do the doctoral course in Physical Activity and Curso de Doutoramento em Atividade Health designed by the Research Centre in Física e Saúde, organizado pelo Centro Physical Activity Health and Leisure de Actividade Física, Saúde e Lazer (CIAFEL), Faculty of Sports - University of (CIAFEL) da Faculdade de Desporto da Porto, according to the Decree-Law Universidade do Porto, nos termos do No.74/2006 from March 24th. Decreto-lei 74/2006 de 24 de Março. Supervisors: Joana Carvalho, PhD Jorge Mota, PhD Porto, 2011 Marques, E. A. (2011). Exercise training, Bone and Aging. Evidence from different impact loading exercise interventions on age-related bone loss. Porto: E. A. Marques. Dissertação de Doutoramento em Atividade Física e Saúde apresentada à Faculdade de Desporto da Universidade do Porto. KEY-WORDS: AGE, PHYSICAL ACTIVITY, BONE DENSITY, FRACTURE RISK, BIOMARKERS, META-ANALYSIS. Old age is like everything else. To make a success of it, you’ve got to start young. Fred Astaire Funding The candidate work was supported by a doctoral grant from Portuguese Foundation for Science and Technology (FCT) SFRH/BD/36319/2007, and the doctoral thesis was included in a financed research project PTDC/DES/102094/2008 FCOMP-01-0124- FEDER-009587 from FCT. Acknowledgments I’m grateful to my supervisor Professor Joana Carvalho for her guidance during the course of this work, and for the support in all moments (far beyond the academic level).