Silvan Smulders BW.Indd
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Aalseth Aaron Aarup Aasen Aasheim Abair Abanatha Abandschon Abarca Abarr Abate Abba Abbas Abbate Abbe Abbett Abbey Abbott Abbs
BUSCAPRONTA www.buscapronta.com ARQUIVO 35 DE PESQUISAS GENEALÓGICAS 306 PÁGINAS – MÉDIA DE 98.500 SOBRENOMES/OCORRÊNCIA Para pesquisar, utilize a ferramenta EDITAR/LOCALIZAR do WORD. A cada vez que você clicar ENTER e aparecer o sobrenome pesquisado GRIFADO (FUNDO PRETO) corresponderá um endereço Internet correspondente que foi pesquisado por nossa equipe. Ao solicitar seus endereços de acesso Internet, informe o SOBRENOME PESQUISADO, o número do ARQUIVO BUSCAPRONTA DIV ou BUSCAPRONTA GEN correspondente e o número de vezes em que encontrou o SOBRENOME PESQUISADO. Número eventualmente existente à direita do sobrenome (e na mesma linha) indica número de pessoas com aquele sobrenome cujas informações genealógicas são apresentadas. O valor de cada endereço Internet solicitado está em nosso site www.buscapronta.com . Para dados especificamente de registros gerais pesquise nos arquivos BUSCAPRONTA DIV. ATENÇÃO: Quando pesquisar em nossos arquivos, ao digitar o sobrenome procurado, faça- o, sempre que julgar necessário, COM E SEM os acentos agudo, grave, circunflexo, crase, til e trema. Sobrenomes com (ç) cedilha, digite também somente com (c) ou com dois esses (ss). Sobrenomes com dois esses (ss), digite com somente um esse (s) e com (ç). (ZZ) digite, também (Z) e vice-versa. (LL) digite, também (L) e vice-versa. Van Wolfgang – pesquise Wolfgang (faça o mesmo com outros complementos: Van der, De la etc) Sobrenomes compostos ( Mendes Caldeira) pesquise separadamente: MENDES e depois CALDEIRA. Tendo dificuldade com caracter Ø HAMMERSHØY – pesquise HAMMERSH HØJBJERG – pesquise JBJERG BUSCAPRONTA não reproduz dados genealógicos das pessoas, sendo necessário acessar os documentos Internet correspondentes para obter tais dados e informações. DESEJAMOS PLENO SUCESSO EM SUA PESQUISA. -

Industry at Eurocmr 2019
EuroCMR2019 EXTENDING THE CLINICAL VALUE OF CMR THROUGH QUALITY AND EVIDENCE 2-4 May Venice ITALY FINAL PROGRAMME 17th Annual Meeting on Cardiovascular Magnetic Resonance (CMR) of the European Association of Cardiovascular Imaging (EACVI) www.escardio.org/EACVI #EuroCMR Programme as of 5 April 2019 Building overview EACVI MEMBERS’ LOUNGE COURSE ROOM MEETING ROOMS POSTERS Level 1 SPEAKER SERVICE CENTRE SALA DARSENA ENTRANCE SALA DARSENA SALA GRANDE Level 0 EXHIBITION REGISTRATION MAIN ENTRANCE Level -1 RAPID FIRE ROOM SALA ZORZI Welcome Letter Dear Friends & Colleagues, Welcome to EuroCMR 2019 Congress in Venice, Italy! We are delighted to welcome you to EuroCMR 2019, the annual meeting on CMR of the European Association of Cardiovascular Imaging (EACVI), which has developed over the last few years into the largest European CMR conference with over 1,000 participants in Prague in 2017. This year’s congress theme is “Extending the clinical value of CMR through quality and evidence”. Throughout the programme, we will focus on the existing and emerging evidence behind the established and potential clinical indications of CMR and will highlight how high-quality research and clinical service provision can make a difference to patients and populations. In times of very limited resources available to finance health care, we will also discuss health economics relevant to cardiac imaging in a multi-modality context. We want this to be your conference. We invited all members of the EACVI to actively contribute to EuroCMR 2019 by responding to our call for session proposals and we look forward to providing an international platform for you to present your research abstracts. -

Author Index
ABSTRACT AUTHOR INDEX The number following the name refers to the abstract number, not the page number. A number in bold indicates the author is the abstract presenter. Aaboe, Kasper 1915-P Adams, Rachel 2308-PUB, 2315-PUB Akhmetova, Saule B. 644-P, 649-P Aadahl, Mette 1997-P Adams, Sara R. 837-P, 1214-P Akhrass, Firas 1250-P Aamodt, Kristie I. 2056-P Adamson, Karen 1036-P, 1123-P, 1164-P, 1170-P Aki, Nanako 2412-PUB Abate, Nicola 33-OR Adeli, Khosrow 103-OR, 106-OR Akiyama, Noriko 1102-P Abbas, Malak 494-P, 2084-P Adhami, Anwar 539-P Akiyama, Yoshitaka 976-P Abbasi, Fahim 2140-PUB Adhikari, Pramisha 1726-P Akkari, Patrick Anthony 1613-P Abbass, Ibrahim 844-P, 2159-PUB Adhikary, Till 1850-P Akkireddy, Padmaja 2236-PUB Abbott, Akshar B. 608-P Adi, Saleh 873-P Akolkar, Beena 819-P Abbott, Alice M. 222-OR Adili, Fatemeh 2194-PUB Akoumianakis, Ioannis 215-OR Abbott, Scott 993-P, 1000-P, 1001-P, 1007-P Adkins, Laura 417-P Akturk, Halis K. 1586-P Abburi, Nandini 6-OR Advani, Andrew 485-P, 501-P, 552-P, 670-P Akueson, Cecelia E. 1347-P Abcouwer, Steven F. 601-P Aeby, Sébastien 766-P Al Jobori, Hussein 1097-P, 1875-P ABSTRACT AUTHOR INDEX Abdallah Hasan, Ahmed 505-P Aerni, Hans R. 1754-P Al Khaldi, Rasha 413-P, 2165-PUB Abdella, Nabilla 413-P, 2165-PUB Affi nati, Alison 2457-PUB Ala-Korpela, Mika 198-OR Abdesselam, Ines 2018-P Afrin, M.S.T. Rejina 2295-PUB Alarcón, Javier 1672-P Abdul-Ghani, Muhammad 188-OR, 1097-P, 1961-P Agarwal, Rishi 943-P Alarcon, Maria Lis 2338-PUB, 2362-PUB Abdulina, Galiya A. -
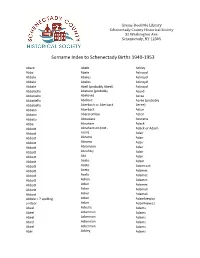
Surname Index to Schenectady Births 1940-1953
Grems-Doolittle Library Schenectady County Historical Society 32 Washington Ave. Schenectady, NY 12305 Surname Index to Schenectady Births 1940-1953 Abare Abele Ackley Abba Abele Ackroyd Abbale Abeles Ackroyd Abbale Abeles Ackroyd Abbale Abell (probably Abeel) Ackroyd Abbatiello Abelone (probably Acord Abbatiello Abelove) Acree Abbatiello Abelove Acree (probably Abbatiello Aberbach or Aberback Aeree) Abbato Aberback Acton Abbato Abercrombie Acton Abbato Aboudara Acucena Abbe Abraham Adack Abbott Abrahamson (not - Adack or Adach Abbott nson) Adair Abbott Abrams Adair Abbott Abrams Adair Abbott Abramson Adair Abbott Abrofsky Adair Abbott Abt Adair Abbott Aceto Adam Abbott Aceto Adamczak Abbott Aceto Adamec Abbott Aceto Adamec Abbott Acken Adamec Abbott Acker Adamec Abbott Acker Adamek Abbott Acker Adamek Abbzle = ? spelling Acker Adamkiewicz unclear Acker Adamkiewicz Abeel Ackerle Adams Abeel Ackerman Adams Abeel Ackerman Adams Abeel Ackerman Adams Abeel Ackerman Adams Abel Ackley Adams Grems-Doolittle Library Schenectady County Historical Society 32 Washington Ave. Schenectady, NY 12305 Surname Index to Schenectady Births 1940-1953 Adams Adamson Ahl Adams Adanti Ahles Adams Addis Ahman Adams Ademec or Adamec Ahnert Adams Adinolfi Ahren Adams Adinolfi Ahren Adams Adinolfi Ahrendtsen Adams Adinolfi Ahrendtsen Adams Adkins Ahrens Adams Adkins Ahrens Adams Adriance Ahrens Adams Adsit Aiken Adams Aeree Aiken Adams Aernecke Ailes = ? Adams Agans Ainsworth Adams Agans Aker (or Aeher = ?) Adams Aganz (Agans ?) Akers Adams Agare or Abare = ? Akerson Adams Agat Akin Adams Agat Akins Adams Agen Akins Adams Aggen Akland Adams Aggen Albanese Adams Aggen Alberding Adams Aggen Albert Adams Agnew Albert Adams Agnew Albert or Alberti Adams Agnew Alberti Adams Agostara Alberti Adams Agostara (not Agostra) Alberts Adamski Agree Albig Adamski Ahave ? = totally Albig Adamson unclear Albohm Adamson Ahern Albohm Adamson Ahl Albohm (not Albolm) Adamson Ahl Albrezzi Grems-Doolittle Library Schenectady County Historical Society 32 Washington Ave. -

Participant List
Participant List 10/20/2019 8:45:44 AM Category First Name Last Name Position Organization Nationality CSO Jillian Abballe UN Advocacy Officer and Anglican Communion United States Head of Office Ramil Abbasov Chariman of the Managing Spektr Socio-Economic Azerbaijan Board Researches and Development Public Union Babak Abbaszadeh President and Chief Toronto Centre for Global Canada Executive Officer Leadership in Financial Supervision Amr Abdallah Director, Gulf Programs Educaiton for Employment - United States EFE HAGAR ABDELRAHM African affairs & SDGs Unit Maat for Peace, Development Egypt AN Manager and Human Rights Abukar Abdi CEO Juba Foundation Kenya Nabil Abdo MENA Senior Policy Oxfam International Lebanon Advisor Mala Abdulaziz Executive director Swift Relief Foundation Nigeria Maryati Abdullah Director/National Publish What You Pay Indonesia Coordinator Indonesia Yussuf Abdullahi Regional Team Lead Pact Kenya Abdulahi Abdulraheem Executive Director Initiative for Sound Education Nigeria Relationship & Health Muttaqa Abdulra'uf Research Fellow International Trade Union Nigeria Confederation (ITUC) Kehinde Abdulsalam Interfaith Minister Strength in Diversity Nigeria Development Centre, Nigeria Kassim Abdulsalam Zonal Coordinator/Field Strength in Diversity Nigeria Executive Development Centre, Nigeria and Farmers Advocacy and Support Initiative in Nig Shahlo Abdunabizoda Director Jahon Tajikistan Shontaye Abegaz Executive Director International Insitute for Human United States Security Subhashini Abeysinghe Research Director Verite -
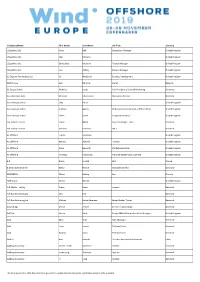
Company Name First Name Last Name Job Title Country
Company Name First Name Last Name Job Title Country 1StopWind Ltd Arran Bell Operations Manager United Kingdom 1StopWind Ltd. Alan Mckerns United Kingdom 1StopWind Ltd. Bernadette McAulay Finance Manager United Kingdom 1StopWind Ltd. Joel Telling General Manager United Kingdom 23 Degrees Renewables Ltd Ed Woodrow Business Development United Kingdom 24SEA bvba Gert De Sitter Owner Belgium 3S Europe GmbH Matthias Lamp Vice President of Sales & Marketing Germany 3sun Denmark ApS Christian Christensen Operations Director Denmark 3sun Group Limited Jody Potter United Kingdom 3sun Group Limited Graham Hacon VP Business Development, Offshore Wind United Kingdom 3sun Group Limited Sherri Smith Company Secretary United Kingdom 3W Industri Service Simon Øland Project manager - sales Denmark 3W Industri Service Kenneth Pedersen IWI-S Denmark 4C Offshore Lauren Anderson United Kingdom 4C Offshore Richard Aukland Director United Kingdom 4C Offshore Rosie Haworth Market Researcher United Kingdom 4C Offshore Vincenzo Poidomani Principal Geotechnical Engineer United Kingdom 8.2 Bruno ALLAIN CEO France 8.2 Monitoring GmbH Bernd Höring Managing director Germany 920338402 Ellinor Meling Ceo Norway A&P Group Emma Harrick United Kingdom A.P. Møller Holding Simon Ibsen Investor Denmark A/S Dan-Bunkering Ltd. Jens Kirk Denmark A/S Dan-Bunkering Ltd. Michael Brunø-Sørensen Senior Bunker Trader Denmark A1wind Aps Martin Jensen Director / A1wind Aps Denmark AAF Ltd Steven Brett Europe MFAS Aftermarket Sales Manager United Kingdom AAG Allan Tarp Sales Manager Denmark -

Müller-Weiss Disease: a Topical Review
FAIXXX10.1177/1071100719877000Foot & Ankle InternationalAhmed et al 877000research-article2019 Topical Review Foot & Ankle International® 2019, Vol. 40(12) 1447 –1457 Müller-Weiss Disease: A Topical Review © The Author(s) 2019 Article reuse guidelines: sagepub.com/journals-permissions DOI:https://doi.org/10.1177/1071100719877000 10.1177/1071100719877000 journals.sagepub.com/home/fai Abdel-Salam Abdel-Aleem Ahmed, MD1 , Mahmoud Ibrahim Kandil, MD1 , Eslam Abdelshafi Tabl, MD1 , and Amr S. Elgazzar, MD1 Level of Evidence: Level V, expert opinion. Keywords: Müller-Weiss disease, Brailsford disease, foot arthrodesis, calcaneal osteotomy Müller-Weiss disease (MWD) is a painful foot condition articulates proximally with the talar head, distally with the characterized by deformity, sclerosis, and fragmentation of cuneiforms, and inferolaterally with the cuboid. Proximally, the navicular. The diseased navicular is characteristically the talocalcaneonavicular articulation is also known as the comma shaped with varying degrees of arthritis present at acetabulum pedis for morphologic similarity to the hip the talonavicular and naviculocuneiform joints.17,19,27 Other joint. It is a ball-socket synovial joint allowing for rotatory names for MWD include Brailsford disease,37 adult tarsal and gliding movements. The talocalcaneonavicular and the scaphoiditis,26 spontaneous adult navicular osteonecrosis,34 naviculocuneiform joints sustain the greatest load transmis- and listhesis navicularis.23 There is substantial controversy sion in comparison to other foot joints.11 regarding the etiology, pathophysiology, and natural history The navicular is the latest tarsal bone to ossify. of MWD, as well as optimal methods of treatment. This Ossification occurs during the period of increased mobility article reviews the historical and current literature regarding and activity in children (at the end of the second year in girls MWD, including the latest surgical interventions that have and at the beginning of the fourth year in boys). -

Dynamic Covalent Polymers for Biomedical Applications Cite This: Mater
MATERIALS CHEMISTRY FRONTIERS View Article Online REVIEW View Journal | View Issue Dynamic covalent polymers for biomedical applications Cite this: Mater. Chem. Front., 2020, 4,489 a b c d Yan Zhang, * Yunchuan Qi, Se´bastien Ulrich, * Mihail Barboiu * and be Olof Ramstro¨m * The rapid development of supramolecular polymer chemistry and constitutional dynamic chemistry over the last decades has made tremendous impact on the emergence of dynamic covalent polymers. These materials are formed through reversible covalent bonds, endowing them with adaptive and responsive features that have resulted in high interest throughout the community. Owing to their intriguing properties, such as self-healing, shape-memory effects, recyclability, degradability, stimuli- responsiveness, etc., the materials have found multiple uses in a wide range of areas. Of special interest is their increasing use for biomedical applications, and many examples have been demonstrated in Received 25th September 2019, recent years. These materials have thus been used for the recognition and sensing of biologically active Accepted 26th November 2019 compounds, for the modulation of enzyme activity, for gene delivery, and as materials for cell culture, DOI: 10.1039/c9qm00598f delivery, and wound-dressing. In this review, some of these endeavors are discussed, highlighting the many advantages and unique properties of dynamic covalent polymers for use in biology and rsc.li/frontiers-materials biomedicine. 1. Introduction a Key Laboratory of Carbohydrate Chemistry and Biotechnology, Ministry of Education, School of Pharmaceutical Sciences, Jiangnan University, Wuxi, 214122, During the last decades, dynamic covalent chemistry has P. R. China. E-mail: [email protected] emerged as a powerful means to generate controlled molecular b Department of Chemistry, University of Massachusetts Lowell, One University Ave., systems and networks, designed self-processes, and complex Published on 03 12 2019. -

ICCA Congress & Exhibition 2007
ICCA Congress & Exhibition 2007 PARTICIPANTS BY SURNAME 2007 Name Company Country , Suharyanto Jakarta Convention & Exhibition Bureau INDONESIA , Farizaludin Jakarta Convention & Exhibition Bureau INDONESIA ABDUL HALIM, Kamilia Malaysia Tourism Promotion Board MALAYSIA Hani ABDUL RAHMAN, Nina Kuala Lumpur Convention Centre MALAYSIA Kartini Bte ABDUL RAHMAN, Malaysia Tourism Promotion Board MALAYSIA Norshamshida ABDUL WAHID, Malaysia Tourism Promotion Board MALAYSIA Norhaida ABU MONASSAR, Anwar Net Conference & Conventions UNITED ARAB EMIRATES ADAMJI, Tasneem African Quest Safaris Ltd. KENYA ADAMS, Barbara Melbourne Exhibition and Convention Centre AUSTRALIA AKKANEEVANICH, Queen Sirikit National Convention Center THAILAND Sasivee ALAGIROVA, Tamara Bedouk Meetings & Events Media FRANCE AL FAHIM, Khalid Dubai International Convention & Exhibition Centre UNITED ARAB EMIRATES AL FALASI, Sultan Dubai Convention Bureau UNITED ARAB EMIRATES AL NUAIMI, Mubarak Abu Dhabi Tourism Authority UNITED ARAB EMIRATES ALONSO, Francisco ExpoChihuahua MEXICO ALTAMURA, Carrie Darwin Convention Centre AUSTRALIA ALTER, Alex ICC Jerusalem International Convention Center ISRAEL ALVEZ SARAVIA, CMP, ICCA Latin America Regional Office URUGUAY Maria José AMARAL, Jose Rui International Conference Center Joaquim Chissano MOZAMBIQUE AMEY, Monique Nice Convention & Visitors Bureau FRANCE AMORNVIVAT, Natwut Thailand Convention & Exhibition Bureau THAILAND AMSEL, Clare Megaron Athens International Conference Centre GREECE ANDERSEN, Peder DIS Congress Service Copenhagen A/S -

Surname Given Age Date Page Maiden Note Abildua Frank 91 22-Dec D-1 Ables Cary James 14 13-Apr D-1 Full Name Abrahamson Ethel 91 9-Mar D-2 Newman Abramson Rose H
Surname Given Age Date Page Maiden Note Abildua Frank 91 22-Dec D-1 Ables Cary James 14 13-Apr D-1 Full name Abrahamson Ethel 91 9-Mar D-2 Newman Abramson Rose H. 81 2-Feb D-2 Accordini Mary G. 70 20-Dec A-13 Ackerman Frances L. 4-May C-5 Adams Eva 83 3-Apr D-2 Stewart Adams Michael J. 76 28-Jan D-1 Adell Alfred S. 48 5-May B-4 Adoba William J., Sr. 64 10-Jan B-6 Agnew W. Lynn 85 12-Sep B-6 Albaugh James 81 9-Oct E-1 Albee Daniel 62 27-Jul D-1 Albert Henrietta 79 13-Apr D-1 Albright Ralph G. 80 13-Apr D-1 Aldrin Myrtle A. 76 15-Jan D-6 Alexander Daniel L. 37 13-Jun C-5 Alexander W. (Rev.) 69 6-Jan D-2 Alexich Jacov "Jack" 60 26-Dec D-1 Allande Arthur 80 13-Oct D-3 Allande Augustina 79 2-May C-2 Allen James P., Sr. 49 30-Oct D-1 Allen Ernest L. 48 6-Sep B-5 Veteran of the Korean conflict Allen Lizzie 3-Jan A-11 Allen William M. 75 21-Mar C-2 Allen Aryln J. 60 5-Jun F-7 Allen Annie 101 4-Feb D-1 Aller Edward D. 81 11-Jan D-2 Almanza Maria 81 13-Feb D-1 Aloia Frank, Sr. 90 9-Feb C-2 Alongi Samuel 70 5-Jan C-5 Veteran of World War II Alpert Samual 97 15-Dec C-7 Alsdorf Rose Evelyn 52 30-Sep C-3 Full name Alsip Yvetta E. -

Activation De La Phosphodiestérase De Type 2 Pour Traiter L'insuffisance Cardiaque
NNT : 2016SACL336 THESE DE DOCTORAT DE L’UNIVERSITE PARIS-SACLAY PREPAREE A UNIVERSITE PARIS SUD ECOLE DOCTORALE N° 569 ITFA Innovation thérapeutique: du fondamental à l’appliqué Spécialité de doctorat: Physiologie, physiopathologie Par Marta Lindner Activation de la phosphodiestérase de type 2 pour traiter l'insuffisance cardiaque Thèse présentée et soutenue à Châtenay-Malabry, le 12 Octobre 2016 Composition du Jury : M. Christian POÜS Professeur, UPSud Président Mme Claire LUGNIER DR Emérite CNRS Rapporteur Mme Catherine PAVOINE CR INSERM Rapporteur Mme Liliana CASTRO Maître de Conférence, UPMC Examinateur M. Ali EL-ARMOUCHE Professeur, Univ. Dresden Examinateur M. Grégoire VANDECASTEELE DR INSERM Examinateur M. Rodolphe FISCHMEISTER DR INSERM Directeur de thèse Table of contents Sommaire Introduction ______________________________________________________________ 13 I. The heart _______________________________________________________________ 14 I.1 Anatomy of the heart ________________________________________________________ 14 I.2 Cardiac excitation-contraction coupling __________________________________________ 15 I.2.1 Structures involved in cardiac ECC _____________________________________________________ 15 I.2.1.1 Sarcomere and T-tubules _______________________________________________________ 15 I.2.1.2 Sarcoplasmic reticulum ________________________________________________________ 17 I.2.1.3 Myofilaments ________________________________________________________________ 17 I.2.1.4 Mitochondria ________________________________________________________________ -
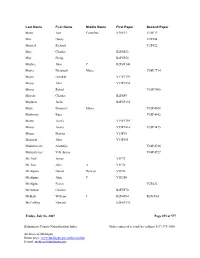
Kalamazoo County Naturalization Index Order Copies of Records by Calling (517) 373-1408
Last Name First Name Middle Name First Paper Second Paper Maus Joos Cornelius V30P33 V30P33 Max Henry V2P204 Maxted Richard V2P422 May Charles B2F6P23 May Philip B2F6P55 Maybee John C B2F6P140 Mayer Elizabeth Marie V58P3714 Mayer Hendrik V17P3359 Mayer John V17P3234 Mayer Robert V51P3086 Mayers Charles B2F6P9 Mayhew Jacob B2F6P135 Mayr Emanuele Maria V62P4308 Mazkrists Ruta V65P4942 Mazur Josefa V19P3789 Mazur Josefa V55P3416 V55P3415 Mazur Marcin V15P18 Mazurak John V13P291 Mazurkevics Anatolijs V64P4726 Mazurkevics Vilhelmina V64P4727 Mc Neil James V5P72 Mc Rae John A V5P76 McAlpine Daniel Duncan V3P26 McAlpine John C V3P250 McAlpine Peeter V2P426 McArthur Charles B2F5P70 McBeth William L B2F6P84 B2F6P84 McCaffrey Edward B2F6P136 Friday, July 06, 2007 Page 299 of 577 Kalamazoo County Naturalization Index Order copies of records by calling (517) 373-1408 Archives of Michigan Home page: www.michigan.gov/archivesofmi E-mail: [email protected] Last Name First Name Middle Name First Paper Second Paper McCall Hannah V16P3084 McCallum Duncan Clyde V10P370 McCallum Duncan Clyke V23P212 V23P212 McCally Geoffrey Thomas V52P3165 McCamby Alex B2F6P1 McCamly Daniel B2F6P5 McCandless Frances Georgina Forsythe V61P4262 McCandless Hugh Milligan V20P4331 McCandry Daniel B2F6P5 McClelland George Charles V23P101 McClure Mary Abernethy V10P389 McClurky Charles B2F6P67 McCormick George W V5P33 McCormick Olga V48P2744 McCray Robert B2F6P157 McCudden John B2F6P150 McCue Hugh B2F5P56 McCue John B2F6P76 McCue Peter V62P4379 McCugan Vincent Murray V19P3955